 |
 |
| Korean J Intern Med > Volume 19(1); 2004 > Article |
|
Abstract
Background :
Maspin is a serpin family of protease inhibitors. Althouth maspin has been considered a tumor suppressor that inhibits the motility, invasion, and metastasis of breast and prostatic cancer cells, there are many conflicting reports about maspin expression and cancer prognosis.
Methods :
To investigate whether the expression of maspin could be used as a prognostic marker in pancreatic cancer, 72 paraffin-embedded pancreatic ductal adenocarcinomas were analyzed using immunohistochemistry. We examined the prognostic value of maspin as well as its relationship with clinicopathological features.
Results :
Maspin expression was observed in all pancreatic ductal adenocarcinoma. Unlike cancer tissues, however, faint or no expression was observed in the corresponding normal pancreatic tissues. In the Cox proportional hazard model, high maspin expression predicted a high hazard rate. Maspin expression had a positive correlation with tumor stage, but there were also no statistically significant relationships between maspin expression and other clinicopathological features.
Maspin (mammary serine protease inhibitor) is a recently identified protein related to the serpin family of protease inhibitors. Maspin expression is detected in normal breast epithelial cells, but is decreased or absent in tumor cells1). Maspin has been known to inhibit tumor cell invasion and metastasis in breast tumor cells2). Pemberton et al. suggested that the down-regulation of maspin may be involved in carcinomas other than in the breast3). But Maass et al. observed that pancreatic cancer revealed different patterns of maspin gene expression from those in breast, prostate, stomach, small intestine, colon, uterus, kidney, and skin cancer cells4). Although, at present, the molecular and biological mechanisms of maspinŌĆÖs functions are unknown, some evidence suggest maspin play a role in pancreatic cancer progression5ŌĆō7).
To the best of our knowledge, there have been no reports concerning maspin expression and its relationship with prognosis in pancreatic cancer. This study was conducted to determine whether the level of maspin expression measured in primary tumors from patients with pancreatic cancers was associated with known prognostic factors and patientsŌĆÖ survival.
Data on 72 pancreatic ductal adenocarcinoma patients had undergone successful resection surgery between 1995 and September 1999 at the Samsung Medical Center were collected. The median age of the patients at the time of operation was 59.8 years (range:16 to 79 years). There were 43 male and 29 female patients. The median period of follow-up was 12.6 months (range; 1ŌĆō66.2 months). Follow-up data were gathered by chart review and prior yearly contact with the patients or the responsible physicians. The patientsŌĆÖ outcomes were verified and updated through the medical record departments of the Samsung Medical Center, as well as through contact with the municipal governments for death certificates when appropriate. The vital statuses of the patients were also determined at the date of the last follow-up while the cause of death was ascertained from the medical record and/or death certificate. The cause of death was classified as secondary to or unrelated to pancreatic cancer. Fifty-six (77.8%) patients died and 16 patients were still alive during the follow-up periods. Twenty-six patients received adjuvant chemotherapy. The most representative paraffin-embedded blocks were chosen for immunohistochemistry by a pathologist. All tumors were staged according to the staging system of the American Joint Committee on Cancer8).
A representative block from each case contained both adequate amounts of tumor tissue and benign non-tumor epithelial cells. 4-╬╝m-thick sections were made, after which they were deparaffinized, and rehydrated. For antigen retrieval, the sections were pretreated in an 800-W microwave oven in a 10 mM citrate buffer, (pH 6.0) for 10 minutes. The primary antibody against maspin (Pharmingen, San Diego, CA, 1:3,000) was applied to the sections for one hour. Then, the sections were incubated with secondary avidin-biotin-complex (LSAB kit, Dako, CA). Diaminobenzidine was used as the chromogen, and hematoxylin as the counterstain. Negative controls were obtained by omitting the primary antibody, while benign breast parenchyma served as the external positive control. The cells were considered positive if granular cytoplasmic/nuclear or cytoplasmic staining was observed3). The total scores were calculated using quick score, where both the proportion and intensity of the cells stained were estimated. This method was also used in breast cancer for scoring the immunostaining signals for the estrogen and progesterone receptors. The proportions were scored as 0 (0), 1 (less than 1/100 of tumor cells), 2 (1/100 to 1/10 of tumor cells), 3 (1/10 to 1/3 of tumor cells), 4 (1/3 to 2/3 of tumor cells), or 5 (more than 2/3 of tumor cells), while the intensity was measured as 0, 1, 2, or 3, corresponding to no staining, weak, moderate, and strong staining, respectively.
A Cox proportional hazards model for the risk ratio was used to assess the simultaneous contribution of the baseline covariate (age, sex, CA19-9, tumor size, location, ECOG performance, state of tumor differentiation, stage, adjuvant chemotherapy, and maspin). The correlation between the total score of maspin expression and clinicopathological features (age, sex, CA19-9, performance, tumor size, location, and state of tumor differentiation) was determined by the SpearmanŌĆÖs rank correlation. A probability of p<0.05 was considered statistically significant.
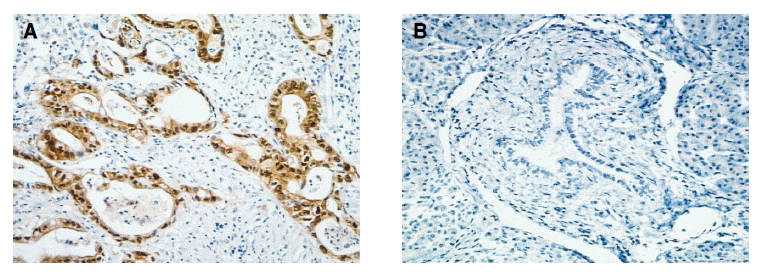
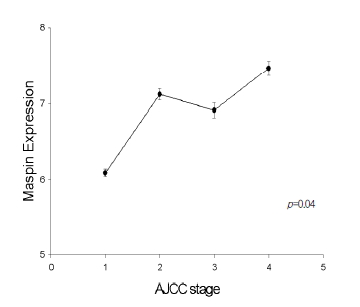
The immunoreactivity of maspin was mainly in cytoplasmic staining, with some in nuclear staining. Maspin expression was observed in all pancreatic ductal adenocarcinoma (Figure 1A) (72/72; 100%). All the pancreatic ductal adenocarcinoma had from five to eight quick scores. Unlike in cancer tissues, corresponding normal pancreatic tissues showed faint or no expression (Figure 1B). Maspin expression correlated positively with increased stages of pancreatic cancer tissue (stage I; 13, stage II; 21, stage III; 13, stage IV; 25) (p=0.04) (Figure 2).
Multivariate analysis was performed according to the Cox proportional hazard model to evaluate the prognostic value of maspin expression after adjustment for other prognostic factors (Table 1). Maspin expression was an unfavorable prognostic factor for patients with pancreatic cancer independent of the stage and other clinicopathological features (p=0.022). According to the results, high maspin expression predicted a high hazard rate. However, other clinicopathological parameters such as age, sex, CA19-9, tumor size, location, ECOG performance, and state of tumor differentiation had no significant correlation with maspin expression.
Maspin is a 42 kDa protein with a sequence related to several members of the serpin (serine protease inhibitor) superfamily9ŌĆō11). The maspin gene was originally isolated from normal mammary epithelium by subtractive hybridization9). Maspin mRNA was also detected in a variety of organs, and immunostaining for maspin displayed expressions in the normal prostate, stomach, small intestine, colon, pancreas, uterus, kidney, and skin4). In cultured human mammary myoepithelial cells, maspin is predominantly a soluble cytoplasmic protein that associates with secretory vesicles and is present at cell surfaces4). The maspin gene has been localized to chromosome 18q21.3-q23 within the same region as the plasminogen activator inhibitor-2 gene (PAI-2), the tumor suppressor gene DCC, SCCA1+2, and BCL-2 gene12, 13).
Althouth maspin has been considered a tumor suppressor that inhibits the motility, invasion, and metastasis of breast and prostatic cancer cells, there are many conflicting reports about maspin expression and cancer prognosis.
Since maspin has been known to be regulated at the transcriptional level3), there are some reports about maspin mRNA expression and cancer. In most human breast cancer cells, maspin mRNA is low or absent although the maspin gene is rarely rearranged or deleted14). Nevertheless, silencing of the maspin gene is observed and the aberrant cytosine methylation and heterochromatinization of maspin promoter is thought to be responsible for silencing the maspin gene3).
But Luppi et al. obtained results different from the results above. They reported that RT-PCR for maspin mRNA was a sensitive assay for the study of circulating potentially neoplastic mammary cells in patients with breast cancers and suggested an association between the presence of circulating maspin positive cells and a higher risk of disease progression15). Another study by Oh et al. also showed that immunorectivity for maspin was higher in lymph node-positive breast carcinomas than in lymph node-negative carcinomas16).
To evaluate the expression of maspin and its relationship with prognosis in pancreatic cancer, we used a paraffin embedded block. We did not examine maspin mRNA expression by Northern blot or RT-PCR. Thus, the possibility of detecting a maspin-like protein homologous to maspin by immunostaining could not be excluded, and objective quantitative assays, such as Northern blot and real time RT-PCR, were not combined. This was a major limitation of our study.
In our immunohistochemistry study, maspin expression was not observed in normal pancreatic tissues, but pancreatic tissues showed increased immunoreactivity with worse prognosis. These results suggested that maspin expression was is upregulated at the pancreatic neoplasm with malignant potential, in contrast to breast and prostate carcinomas, consistent with the results by Maass et al4). In their study, maspin was expressed in 23 of 24 pancreatic tumor specimens, while no expression was observed in all normal pancreatic tissues using Northern blot analysis and immunohistochemistry4).
Although maspin is a serpin family of protease inhibitors, maspin has been known not to have protease-inhibiting activity2, 3). Most protease inhibitors have the ability to prevent tumor invasion and metastasis11). According to a study by Hall, increased maspin expression was combined with aberrant proteinase expression, extracellular matrix degradation, and tumor cell locomotion, with a potential to lead to invasive metastatic cancer17).
Types of human cancer known to have prognostic correlation with maspin expression are mammary carcinoma and oral squamous cell carcinoma. High tumoral maspin expression is associated with improved survival of patients in these cancers18, 19). In contrast to the observations that maspin suppressed the invasion and metastasis of human cancer, we found that all of the pancreatic ductal adenocarcinomas were positive for maspin and that there was a significant positive correlation between maspin expression and clinical stage. Furthermore, high maspin expression predicted unfavorable prognosis according to the Cox proportional hazard model.
Our study suggested maspin expression to have biological relevance in the progression of pancreatic cancers, with potential use as a prognostic marker for pancreatic neoplasm with epithelial origin.
Figure┬Ā1.
Immunohistochemical staining for maspin. (A) Invasive ductal adenocarcinoma of the pancreas with strong cytoplasmic staining of the tumor cells was shown (H&E, ├Ś100). (B) Normal pancreatic tissue of the control cases with negatively stained acinar and ductal cells was shown (H&E, ├Ś100).
Figure┬Ā2.
Positive correlation between maspin expression and AJCC stage. High maspin expression was seen in advanced stage. p value=0.04.
Table┬Ā1.
Cox-proportional Hazard Model for the Multivariate Analysis of Prognostic Factors in Pancreatic Cancer
REFERENCES
1. Zou Z, Anisowicz A, Hendrix MJ, Thor A, Neveu M, Sheng S, Rafidi K, Seftor E, Sager R. Maspin, a serpin with tumor suppressing activity in human mammary epithelial cells. Science 263:526ŌĆō5291994.


2. Seftor RE, Seftor EA, Sheng S, Pemberton PA, Sager R, Hendrix MJ. Maspin suppresses the invasive phenotype of human breast carcinoma. Cancer Res 58:5681ŌĆō56851998.

3. Pemberton PA, Tipton AR, Pavloff N, Smith J, Erickson JR, Mouchabeck ZM, Kiefer MC. Maspin is an intracellular serpin that partitions into secretory vesicles and is present at the cell surface. J Histochem Cytochem 45:1697ŌĆō17061997.


4. Maass N, Hojo T, Ueding M, Luttges J, Kloppel G, Jonat W, Nagasaki K. Expression of the tumor suppressor gene maspin in human pancreatic cancers. Clin Cancer Res 7:812ŌĆō8172001.

6. Maass N, Hojo T, Zhang M, Sager R, Jonat W, Nagasaki K. Maspin: a novel protease inhibitor with tumor-suppressing activity in breast cancer. Acta Oncol 39:931ŌĆō9342000.


7. Abraham S, Zhang W, Greenberg N, Zhang M. Maspin functions as tumor suppressor by increasing cell adhesion to extracellular matrix in prostate tumor cells. J Urol 169:1157ŌĆō11612003.


8. Fleming ID, Cooper JS, Henson DE. Exocrine pancreas. AJCC Manual for Staging of Cancer. 5th ed. 121ŌĆō127Philadelphia: Lippincott Raven, 1997.
9. Sheng S, Pemberton PA, Sager R. Production, purification and characterization of recombinant maspin proteins. J Biol Chem 269:30988ŌĆō309931994.


10. Potempa J, Korzus E, Travis J. The serpin superfamily of proteinase inhibitors: structure, function and regulation. J Biol Chem 269:15957ŌĆō159601994.


11. Sager R, Sheng S, Pemberton P, Hendrix MJ. Maspin: a tumor suppressing serpin. Curr Top Microbiol Immunol 213:51ŌĆō641996.


12. Schneider SS, Schick C, Fish KE, Miller E, Pena JC, Treter SD, Hui SM, Silverman GA. A serine protease inhibitor locus at 18q21.3 contains a tandem duplication of human squamous cell carcinoma antigen gene. Proc Natl Acad Sci U S A 92:3147ŌĆō31511995.



13. le Beau MM, Overhauser J, Strab RE, Silverman G, Gilliam TC, Ott J, OŌĆÖConnell P, Franncke U, Geurts van Kessek A. Report of the first international workshop on human chromosome 18 mapping. Cytogenet Cell Genet 63:78ŌĆō961993.


14. Domann FE, Rice JC, Hendrix MJ, Futscher BW. Epigenetic sliencing of maspin gene expression in human breast cancers. Int J Cancer 85:805ŌĆō8102000.


15. Luppi M, Morselli M, Bandieri E, Federico M, Marasca R, Barozzi P, Ferrari MG, Savarino M, Frassoldati A, Torelli G. Sensitive detection of circulating breast cancer cells by reverse-transcriptase polymerase chain reaction of maspin gene. Ann Oncol 7:619ŌĆō6241996.


16. Oh YL, Song SY, Ahn G. Expression of maspin in pancreatic neoplasms: application of maspin immunohistochemistry to the differential diagnosis. Appl Immunohistochem Mol Morphol 10:62ŌĆō662002.


17. Hall AK. Differential expression of thymosin genes in human tumors and in the developing human kidney. Int J Cancer 48:672ŌĆō6771991.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



