 |
 |
| Korean J Intern Med > Volume 1(1); 1986 > Article |
|
Abstract
To assess the role and responses of lipoperoxide and superoxide dismutase in hypertensive disease, the serum and erythrocyte lipid peroxide and erythrocyte superoxide dismutase levels were measured in 65 normal persons and hypertensive patients.
The results are summerized as follows:
In normal persons, serum and erythrocyte lipoperoxide values showed an increase according to age, but the elevation was not statistically significant.
In normal persons, the erythrocyte superoxide dismutase value did not change according to age.
In hypertensive patients, the serum and erythrocyte lipoperoxide values showed increases of 10.8% and 26% respectively compared to those of the controls.
In hypertensive patients, the erythrocyte superoxide dismutase value showed a decrease of 29.9% compared to that of the controls.
Glavid1) reported that lipoperoxide produced in vivo is related to atherosclerosis. We proposed to examine the relationship between the concentration of serum and tissue lipoperoxide with damage to tissue. Recently, it has been noticed that lipoperoxide induces various disease including atherosclerosis.2ŌĆō5)
These lipoperoxides are produced by a chain reaction between superoxide radicals and unsaturated fatty acids. Lipoperoxide inhibits production of prostaglandin l2 in vascular endothelium and causes cell membrane degeneration in smooth muscle cells by polymerization with protein. It is these actions that are thought to be the mechanism behind the development of atherosclerosis.
We investigated the difference between serum and R.B.C. lipoperoxide and the degree of activation of superoxide dismutase in 38 normal controls who had no evidence of atherosclerosis, either symptomatically or by investigation, and 27 hypertensive patients admitted to the Dept. of Internal Medicine, College of Medicine, Chosun University. We report our experimental results with a review of the literature.
The study involved 2 groups. The control group consisted of 38 hospital staff who showed no evidence of atherosclerotic disease in the general medical check-up. The experimental group consisted of 27 hypertensive patients admitted to the medical department of the Chosun University Hospital, who had not previously been prescribed any drugs that interact with lipid metabolism (Table 1).
Among chemical reagents, ferricytochrome C, xanthine oxidase, xanthine, thiobarbituric acid (T.B.A) and 1,1,3,3-tetraethoxy propane (T.E.P) were purchased from Sigma Chemical Co., St Louise Missouri, U.S.A. E-Merck made E.D.T.A., ammonium sulphate, potassium chloride, potassium phosphate, phospotungstic acid, ethanol and chloroform. Hayashi made sodium dodecyl sulphate and Ishisu made isobutanol.
Collection of blood samples: 3.5ml. of venous blood was taken from all subjects after having been starved for more than 12 hours over-night. The serum was immediately separated by centrifugation.
1) Measurement of lipoperoxide
(A) Serum lipoperoxide: We followed the method of Yagi.8,9) 50╬╝l of serum was accurately pipetted into centrifuge tubes. 4ml. of 1/12N H2SO4 and 0.5ml. of 10% phosphotungstic acid were added and mixed thoroughly. The solutions were left at room temperature for 5 minutes and centrifuged at 10┬░C for 10 minutes at 4,000 r.p.m.
The supernatant was discarded and the precipitate was washed twice by the above mentioned procedure. To the obtained precipitate, 4ml. of distilled water was added yielding a cloudy solution and 1 ml. of T.B.A. reagent solution (0.67% T.B.A. sol. + acetic acid 0.5/0.5% v/v).
The samples were then boiled for 60 minutes at 95┬░C. After boiling, the test tubes were cooled under running water. To the extract 0.5ml. of n-butanol was added and was shaken vigorously. The n-butanol layer was separated by centrifugation for 10 minutes at 4,000 r.p.m. and the absorbtion was measured at 532nm. in a Shimadzu dual difference spectrofluorphotometer. Lipoperoxide fluorescence was compared to standard solutions of 4ml. T.E.P. (0.55 Mol/ml.).
(B) R.B.C. lipoperoxide: 3.5ml. of blood samples was centrifuged at 4┬░C after the serum was removed. The cells deposited were treated as follows: 0.9% of normal saline was added in equal volumes to the sediment. The samples were centrifuged for 15 minutes and supernatant discarded. The sediment was treated 3 times in this way to remove water, and ethanol and chloroform solution added in order to precipitate hemoglobin and obtain R.B.C. cytoplasm. To 0.5ml. of the cytoplasmic solution obtained 0.2ml. of 7% sodium dodecyl sulphate solution was added.
Lipoperoxide in R.B.C. cytoplasm was then estimated by the same method as for serum. Sample concentrations were determined from the standard curve of T.E.P., standard over the range of 1ŌĆō5 nMol/ml.
2) Measurement of superoxide dismutase in R.B.C.: R.B.C. cytoplasm was obtained by the same process as used in the estimation of cytoplasmic lipoperoxide concentrations. The activity of the cytoplasmic superoxide dismutase was estimated by the following method: Xanthine oxidase will produce superoxide radicals from its substrate xanthine. These superoxide radicals will inhibit the reduction of ferricytochrome C. In the prescence of R.B.C. cytoplasm this inhibition will be reduced by utilization of the superoxide radical by any superoxide dismutase present in the cytoplasm. 2.3ml. of 50 mM phosphate buffer (pH 7.8) and 0.3ml. of 0.5mM xanthine are well mixed in a 3ml. cuvette. This was maintained at 25┬░C in a water bath and 0.1 ml. of xanthine oxidase soution whose activity produced an increase in the light absorbance at a rate of 0.020/min was added. In a second cuvette, we added cytoplasmic coenzyme and recorded the change in light absorbtion. We designated 1 unit as that activity which resulted in a 50% inhibition of the ferricytochrome C reduction rate of the control.
In the control group, the serum lipoperoxide values were 2.41 ┬▒ 0.53 nMol/ml in males and 2.35 ┬▒ 0.39nMol/ml in females. There was no significant difference between the sexes (p>0.1). The erythrocytes lipoperoxide value in men and women were 1.80 ┬▒0.55 nMol/ml and 1.71 ┬▒ 0.62 nMol/ml respectively and again there was no significant difference between the sexes (p>0.1).
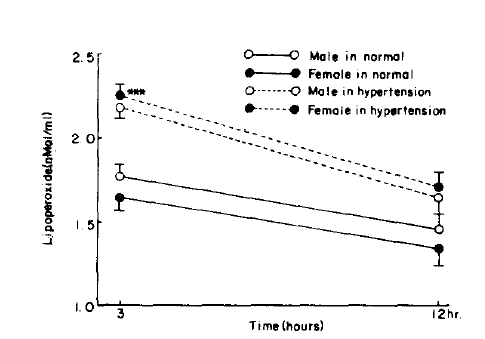
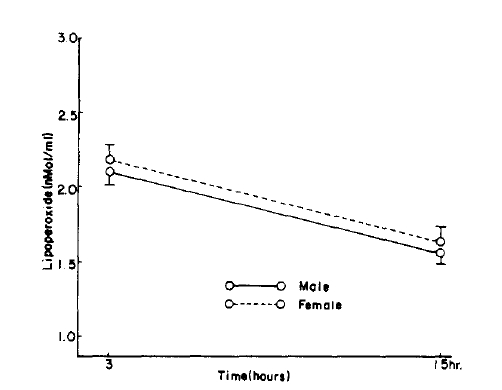
When divided by age, the control serum lipoperoxide values were 2.22 ┬▒ 0.43 nMol/ml for the 10ŌĆō39 age group and 2.68 ┬▒ 0.48 nMol/ml for the 40ŌĆō70 age group, and erythrocyte lipoperoxide values were 1.63 ┬▒ 0.67 nMol/ml and 1.77 ┬▒ 0.59 nMol/ml for the 10ŌĆō39 and 40ŌĆō70 age group respectively. Although the trend shows increasing concentration with age they were not statistically significant (Table 2, Fig. 1, 2). In the hypertensive patient group, the serum lipoperoxide values were 3.25 ┬▒ 0.41 nMol/ml in men and 3.36 ┬▒ 0.68 nMol/ml women, and erythrocyte lipoperoxide values were 2.10 ┬▒ 0.50nMol/ml in men and 2.18 ┬▒ 0.51 nmol/ml in women.
These represented an increase in the mean values of the combined sexes of 10.8% in serum and 26% in erythrocyte lipoperoxide values (Table 2, Fig. 3ŌĆō6).
In normal persons superoxide dismutase values were 13.46┬▒1.61 units/ml for the 10ŌĆō39 age group and 13.24┬▒1.68 units/ml for the 40ŌĆō70 age group and were not significantly different. But in the hypertensive patients the erythrocyte superoxide dismutase values showed a decrease of 29.9% compared to those of the controls (p<0.005) (Table 3).
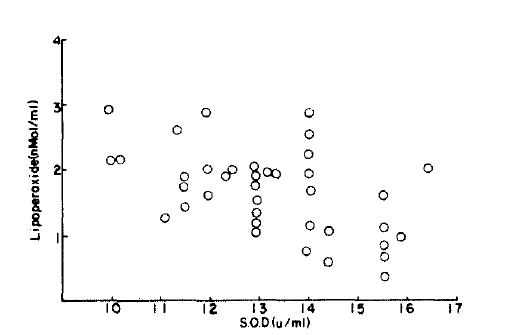
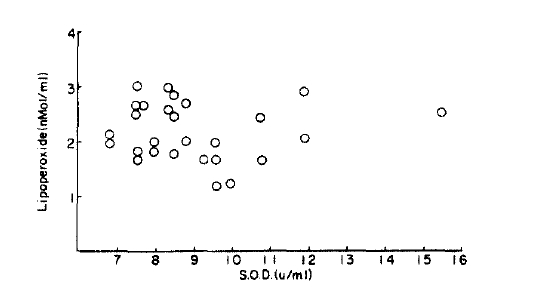
There was a correlation between lipoperoxed and superoxide dismutase (r=0.02) but this was not significantly different from the normal control group (p<0.1) (Fig. 7, 8).
In normal persons superoxide dismutase values were 13.46 ┬▒ 1.61 units/ml for the 10-39 age group and 13.24 + 1.68 units/ml for the 40-70 age group and were not significantly different. But in the hypertensive patients the erythrocyte superoxide dismutase values showed a decrease of 29.9% compared to those of the controls (p<0.005) (Table 3).
It has recently been reported that blood lipoperoxide concentration are related to various diseases including atherosclerosis.2ŌĆō5) There are two mechanisms by which lipoperoxide may be increased in different diseases. First, synthesis of large amounts of lipoperoxide from the specific diseased organ is released into the blood and secondly, it may be produced in the blood by auto-oxidation of blood lipids.
Unsaturated fatty acid has been recommended rather than cholesterol rich saturated fatty acid to patients with atheroscerosis and hyperlipidemia. However, Chun et al.13,14) said that unsaturated fatty acid was a factor in precipitating atherosclerosis due to the production of lipoperoxide from it. Sun Chun et al.5,7) reported that increased lipoperoxide in blood decreased synthesis of prostaglandin l2 in vascular endothelial cells with subsequent stimulation of platelet aggregation which increased thromboxane A2 that damaged vascular endothelium, together with the formation of large quentities of L.D.L.
It was also reported that the protein of endothelial and smooth muscle media cells is denatured by conjugation with lipoperoxide resulting in damage to their cell membranes.
Inhibition of lipoperoxide synthesis and or removal of lipoperoxide present, prevented cell membranes from the toxic effects. Henry et al.15,16,17) announced that vitamin E, a quinine derivative, phenol, superoxide dismutase, peroxide and catalase were antioxidants that decreased lipoperoxide concentration in blood.
Michelson et al.18,19) reported that superoxide radicals react with thiol groups and tryptophan in cells with a fatal result in human beings. Petrone et al.20) stated that superoxide radicals produced by neutrophils reacted with a precurssor extracellularly to produce neutrophil chemotactic factor (N.C.F.)
McCord et al.21,22,23) reported that the harmful effect of the superoxide radicals was lost after it was converted to H2O2 by superoxide dismutase and that this enzyme was present in every cell of organs utilizing oxygen, preventing the toxicity caused by the presence of the superoxide radical.
Willis24,25) reports that some cells utilizing triplet oxygen produce intermediate metabolites such as the superoxide radicals. H2O2. singlet oxygen and the hydroxyl radical. The superoxide radical is highly active in performing an important role in the bactericidal process of phagocytes. However, superoxide radicals cause injury to living tissue by promoting lipoperoxide reactions with enzymes and nucleic acid. Hassan 21,26,27,28). Fridovich16,17,29ŌĆō32) reports that there are scavenger enzymes such as superoxide dismutase, catalase, and peroxidase to clear the harmful effects of oxygen metabolites from the blood. Originally, superoxide dismutase was extracted from ox R.B.C. and liver tissue as cupriferous, hemocupiein or hepatocuprein by Mann and Keilm.33)
Recently Yagi,8,9) has reported the significance of measuring lipoperoxide concentrations in the diagnosis of heart disease of measuring lipoperoxide concentrations in the diagnosis of heart disease and geriatric disease, since the development of the T.B.A. fluorescence methods which can now estimate the very small concentrations of lipoperoxide present in the blood. Yoo13,14,37,38,39) suggests that atherosclerosis, myocardial infarction and C.V.A. may be prevented by changing the fatty acid intake which will reduce hyperlipidemia and platelet aggregation.
In this study the hypertensive group had significantly higher (10.8%) lipoperoxide concentration than the controls, 3.31 ┬▒ 0.54 and 2.45 ┬▒ 0.46 nMol/ml respectively (p<0.005). This result is in accordance with the work of Chun.39)
In view of the increased lipoperoxide in serum and a decrease in superoxide dismutase in R.B.C. in the hypertensive patients, superoxide dismutase is considered to be a scavenger for oxygen derivatives. Because many factors affect lipoperoxide and superoxide dismatuase further study is needed on their use in the diagnosis of heart and geriatric disease.
Fig.┬Ā1.
Serum lipoperoxide in control according to age, sex, hours. Figure is graphed as mean┬▒S.D. for each time period.
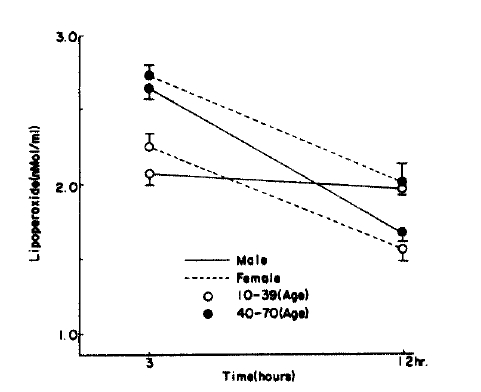
Fig.┬Ā2.
Time course of changes in RBC lipoperoxide in control. Figure is graphed as mean┬▒S.D. for each time period. P value is compared with control group. ***= p<0.005
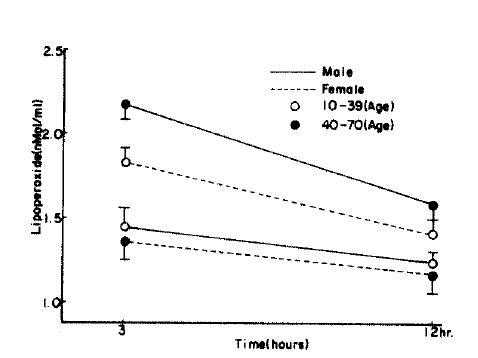
Fig.┬Ā5.
Serum lipoperoxide in control and hypertension P value is compared with control group. ***=p<0.005
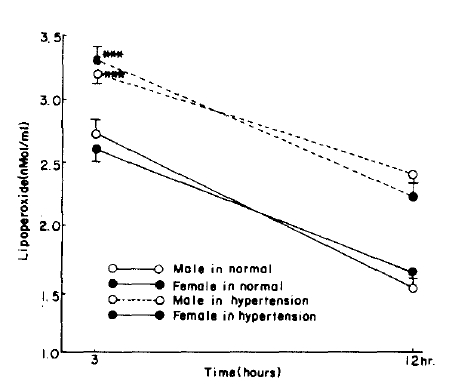
Fig.┬Ā6.
RBC lipoperoxide level in control and hypertension. P value is compared with control group. ***=p<0.005
Table┬Ā1.
Age and sex distribution
| Group | Age | Sex | No. (%) |
|---|---|---|---|
| Normal | 10ŌĆō39 | M | 9 |
| F | 9 | ||
| Total | 18(27.7) | ||
| 40ŌĆō70 | M | 10 | |
| F | 10 | ||
| Total | 20(30.7) | ||
|
|
|||
| Hypertension | 40ŌĆō70 | M | 11 |
| F | 16 | ||
| Total | 27(41.6) | ||
|
|
|||
| Total average(%) | M | 30(46.2) | |
| F | 35(53.8) | ||
| Total | 65(100.0) | ||
Table┬Ā2.
Lipoperoxide level of blood in control and hypertension
| Group | Age | Sex | No. |
Serum lipoperoxide (nMol/ml) mean┬▒S.D.
|
RBC lipoperoxide (nMol/ml) mean┬▒S.D.
|
||
|---|---|---|---|---|---|---|---|
| 3hrs | 12hrs | 3hrs | 12hrs | ||||
| Normal control | 10ŌĆō39 | M | 9 | 2.13┬▒0.49 | 1.95┬▒0.58 | 1.40┬▒0.55 | 1.27┬▒0.59 |
| F | 9 | 2.30┬▒0.39 | 1.63┬▒0.41 | 1.85┬▒0.73 | 1.39┬▒0.45 | ||
| total | 18 | 2.22┬▒0.43 | 1.79┬▒0.51 | 1.63┬▒0.67 | 1.33┬▒0.52 | ||
| 4ŌĆō70 | M | 10 | 268┬▒0.57 | 1,70┬▒0.48 | 2.16┬▒0.22 | 1.54┬▒0.47 | |
| F | 10 | 2.69┬▒0.41 | 1.85┬▒0.39 | 13.9┬▒0.60 | 1.25┬▒0.56 | ||
| total | 20 | 2.68┬▒0.48 | 1.87┬▒0.43 | 1.77┬▒0.59 | 1.39┬▒0.52 | ||
| Average | M | 19 | 2.41┬▒0.53 | 1.82┬▒0.53 | 1.80┬▒0.55 | 1.41 ┬▒0.53 | |
| F | 19 | 2.49┬▒0.39 | 1,75┬▒0.40 | 1.61┬▒0.69 | 1.32┬▒0.50 | ||
| total | 38 | 2.45┬▒0.46 | 1.83┬▒0.47 | 1.70┬▒0.62 | 1.37┬▒0.51 | ||
| Hypertension | 4ŌĆō70 | M | 11 | 3.75┬▒0.40 | 2.15┬▒0.46 | 2.10┬▒0.50 | 1.62┬▒0.48 |
| F | 16 | 3.36┬▒0.68 | 2.11┬▒0.40 | 2.18┬▒0.50 | 1.62┬▒0.37 | ||
| total | 17 | 3.31┬▒0.54*** | 2.13┬▒0.42 | 2.15┬▒0.49* | 1.62┬▒0.41 | ||
Table┬Ā3.
Superoxide dismutase activity control and hypertension
| Group | Age | Sex | No. | S.O.D. activity (╬╝ml) |
|---|---|---|---|---|
| 10ŌĆō39 | M | 9 | 13.99┬▒1.51 | |
| F | 9 | 12.84┬▒1.56 | ||
| Total | 18 | 13.42┬▒1.61 | ||
|
|
||||
| Normal | 40ŌĆō70 | M | 10 | 12.44┬▒1.75 |
| F | 10 | 14.04┬▒1.21 | ||
| Total | 20 | 13.24┬▒1.68 | ||
|
|
||||
| Total | M | 19 | 13.18┬▒1.78 | |
| Average | F | 19 | 13.47┬▒1.48 | |
| Total | 38 | 13.32┬▒1.62 | ||
|
|
||||
| Hypertension | 40ŌĆō70 | M | 11 | 9.03┬▒30 |
| F | 16 | 9.42┬▒2.13 | ||
| Total | 27 | 9.26┬▒1.82*** | ||
REFERENCES
1. Glavind J, Hartman S, Clennesen J, Jessen DE, Dam H. Studies on the role of lipoperoxides in human pathology. Acta Pathol Microb Scand 30:1. 1952.

2. DiGirolamo M, Schdant RC. Etiology of coronary atherosclerosis. The Heart. In: Hurst JW, ed. McGraw-Hill Book Company, 1106. 1978.
3. Hammer CT, Wills ED. The role of lipid composition of the rat liver endoplasmic reticulm and lipid peroxidation. Biochem J 174:855. 1978.
4. Ho S. The trend of Lipoperoxide in arteriosclerotics. Sai-Shin Igaku 36:659. 1981.
5. Izumida H, Yasugi T, Shimizu T, Kobayashi I, Imauo H, Tochilhara T, Tabuchi S, Kishi H. Arterial damages and changes in serum lipids caused by administration of lipoperoxides. Arteriosclerosis 8:295. 1980.
6. Tappel AL. Lipid peroxidation damage to cell components. Federation Proceed 32:1870. 1973.
7. Ho S, Yamamoto M. Mijazakik: A study on arteriosclerosis and lipoperoxides V, about lipoperoxide in the histology of experimental arteriosclerosis. Arteriosclerosis 8:303. 1980.
9. Yagi K. A simple fluometric assay for lipoperoxide in blood plasma. Vitazen 49:403. 1975.
10. Crapo JD, McCord JM, Fridovich I. Preparation and assay of superoxide dismutase. Enzymology. In: Fleisher S, Packer L, eds. Academic press, L:382ŌĆō2931978.

11. McCord JM, Keele BB Jr, Fridovich I. An enzyme based theory of obligate anaerobiosis The physiological function of superoxide dismutase. Proc Natl Acad Sci USA 68:1024ŌĆō10271971.



12. McCord JM, Fricovich I. Superoxide dismutase. Enzymatic function for erythrocup rein(hemocuprein). J Biol Chem 244:6049. 1969.

13. Chun Se Yeol. lipoperoxide and disease. Medical postgraduates (6):9:1981.
14. Chun Hyun Ja, Baeg Tae Hong, Kim Jae Kuk, Chun Se Yeol. A quantitative analysis for lipoperoxide in blood of patient with adult disease. Korean Biochem. Papers Presention 1981.
15. Murray Henry W, Nathan Carl F, Cohn Zanvil A. Macrophage oxygen dependent antimicrobial activity. IV. Role or Endogenus Scavengers of oxygen Intermediates. J Exp Med 153:1610. 1980.



16. Mizuno D, Hayaishi O, Uchiwada C. Live body and oxygen. Asakura 1976.
17. Goto Y. Hypercholesterolemia Igakushoin. 1981.
20. Petrone WF, Lish DK, McCord JM. Free radicals and inflammation, superoxide dependent activation of a neutrophii chemotactactic factor in plasma. Proc Natl Acad Sci USA 68:1024. 1971.



21. Hassan HM, Fridovich I. Enzymatic defenses against the toxicity of oxygen in Escherichia coli. J Bacterial 129:1574. 1977.

22. McCord JM, Fridovich I. The reduction of cytochrome C by Milk xanthine oxidase. J Biol Chem 243:5753. 1978.

23. McCord JM, Fridovich I. The utility of superoxide dismutase in studying free radical reactions. J Biol Chem 243:1373. 1970.

24. Koster Johan F, Slee Regina G. Biochimica et Biophysica Acta 620:489. 1980.
25. Wills ED. The effect of inorganic iron on the thiobarbituric acid method for the determination of lipid peroxides. Blochim Biophys Acta 84:475. 1964.

26. Copps Petkau P P, Kelly K. Superoxide dismutase within the Bovine erythrocytemembrane. Biochemica et Biochimica. Acta 645:71. 1981.

27. Harris J Leuan, Anffret Anthony D, Northrop Frederick D, Walker John E. Structural comparisons of superoxide dismutates. Eur J Biochem 106:297. 1980.


28. Choi Moon Sik, Kweon Yeon Su, Lee Hee Sung. Purification and property of superoxide dismutase of the Rabbit brain. Biochem Dept College of Medicine, Jung ang Univ Jung ang medical newspaper 7:4. 1982.
30. Kellogg EW, Fridovich I IV. Superoxide, Hydrogen peroxide and singlet oxygen in lipid peroxidation by a xanthine oxidase system. J Biol Chem 250:649. 1975.

31. Song Hyun Gon. A study on superoxide dismutase activity in the paraquat damaged rat lung Chosun graduate school medical doctorate papers. 1982.
32. Lee Jeung Yeon, Kweon Yeon Su, Lee Hee Sung. Distribution of superoxide dismutase in the mouse intestine medical newspaper. Jung ang Univ 8:1983.
34. Oishi S. Measurement of lipoperoxide. Saishin Igaku 33:660.
35. Park Jung Sik, Choi Yoon Sik, Lee Yoo Young. Study on serum lipoperoxide of normal Korean and a variety of diseases. Circulatory System 9:1979.
36. You Se Hwa. Study on serum lipoperoxide of normal Korean and otherŌĆÖs circulatory diseases Seoul graduate school medical doctorate papers. 1980.
37. Chun Se Yeol, Kim Jae Kuk. Special lecture and paper of Korean biochemistry seminar. 1981.
-
METRICS

-
- 1 Crossref
- 0 Scopus
- 8,576 View
- 61 Download
- Related articles
-
Plasma Apolipoproteins and Lipids in Normal Persons and Patients with Hypertension1986 July;1(2)




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


