DISCUSSION
Making a diagnosis of carcinoma of the pancreas is very difficult because the pancreas is located in the retroperitoneum, symptoms are diverse, and physical findings not specific. Although recently, ERCP, ultrasonography (US), and computed tomography (CT) have been widely used for the diagnosis of pancreatic cancer, early diagnosis is still difficult. The prognosis of pancreatic cancer is very poor because the retroperitoneal location of the cancer is unsuitable for direct palpation, the proximity of the portal vein, hepatic, and superior mesenteric arteries may preclude removal, depending on the location of the tumor even in its early stage, and the profuse lymphatics and venous drainage of the pancreas invite early and widespread dissemination of the tumor cells. Gudjonssen et al, reported that the absolute 5 years survival rate calculated from 61 clinical studies representing 15,000 patients is 0.4%.
4)
Carcinoma of the pancreas has apparently increased in frequency, perhaps accounting for a fivefold increase mortality rate from carcinoma of the pancreas in Japan during the past 20 years.
5) In the United States the age-adjusted mortality rate from carcinoma of the pancrease has risen from 2.9 to 9.0 per 100,000 population between 1920 and 1970.
6,7) In Korea, the incidence of carcinoma of the pancreas is reported to account for 0.2 to 2.63% of all malignancies.
8ŌĆō10)
The diagnostic methods used for pancreatic disease were divided into pancreatic function tests, immunologic tests and morphologic test.
11) As morphologic tests, simple X-ray of the abdomen, hypotonic duodenography,
13) angiography, radioactive isotope scanning, and percutaneous transhepatic cholangiography have been applied but unfortunately have proved unsatisfactory.
12ŌĆō21) Recently, ERCP, ultrasonography,
14,15,22,39,40) and computed tomogrphy
15,23,39,41ŌĆō44) have been advocated as major innovations in methods for evaluating patients with cancer of the pancreas. ERCP, especially, with the rapid development of fiberoptic duodenoscopes and special techniques, has made it possible to evaluate the diseases of pancreatic and biliary ducts by visualization of the duodenum and ampulla of Vater, direct cannulation for the injection of contrast material into both pancreatic and biliary ducts for radiographic visualization, and by the obtaining of pure pancreatic juice for cytology and chemical analysis.
24)
The success rate of opacification of ducts has been reported to be from 83 to 98.7%, and the rate increases with the advancement of techniques.
27,28,32,35) In our study, of the 122 cases of pancreatic carcinoma, the success rate of cannulation through the ampulla of Vater was 97.5%, the success rate of opacification of the duct 92.6%, and the success rate of visualization of the pancreatic duct, 91.1%. Of the 8 cases with nonvisualized pancreatic ducts, 7 were diagnosed by surgery and other methods as having pancreatic cancer. Therefore, in these cases nonvisualization of the pancreatic duct had diagnostic significance in itself. Thus, the failure rate of visualization of the pancreatic duct was 3.3%.
The diagnostic accuracy of ERCP for disease of the pancreas varies from 80 to 97% depending on the authors.
32,37) Hatfield et al, have reported that diagnostic accuracy in pancreatic carcinoma was 65% (18 patients) by ERCP alone, 54% (14 patients) by pure juice cytology alone, and 92% (24 of 26 patients) when ERCP and cytology were combined.
24) The diagnostic accuracy of ERCP in pancreatic carcinoma was reported as 75% by Ralla et al.
37) and 96% by Freeny et al.
20) Silvis et al.
35) reported that in 43 patients with pancreatic carcinoma, there were 40 successful studies in which the diagnosis could be made in 37 cases by ERCP. Cotton et al.
23) reported that in 14 patients there were 13 successful studies in which the diagnosis could be made of all cases. Freeny et al.
36) reviewed 40 ERCP examinations retrospectively to evaluate the accuracy and reliability of ERCP in the diagnosis of pancreatic carcinoma, and reported that all cases of carcinoma were diagnosed correctly with no false positives and negatives, and concluded that ERCP is a reliable method in diagnosing pancreatic carcinoma. Fitzgerald et al.
15) reported that ERCP diagnosed correctly 8 of 11 cases (73%) of cancer with false negatives in 3 case (27%) and false positives in 3 of 14 cases (21%). Malgelada reported that sensitivity of ERCP in pancreatic cancer was 95%; specificity 90%; positive predictability, 87%; and negative predictability, 97%. In our study, 107 of the 122 cases of pancreatic cancer were diagnosed by the findings of pancreatic ducts showing a diagnostic accuracy of 87.7%. Seven of the 8 cases with nonvisualized pancreatic ducts were diagnosed as having pancreatic cancer by surgery and other methods. In these cases the fact that the pancreatic ducts were not visualized had diagnostic significance in itself, thus the diagnostic accuracy was 93.4%. Of the 111 cases with visualized pancreatic ducts, 107 cases showed abnormal findings and 4 cases of normal pancreatic ducts which were proved to contain carcinoma in the tail (2 cases) and in an uncinate process (2 cases). Thus findings, the diagnostic accuracy of ERCP in pancreatic cancer was 96.4% and the incidence of false negative 3.6% among the cases in which the pancreatic duct was visualized.
According to the results of a comparison of the diagnostic accuracy of current diagnostic tests (ERCP, US and CT) for pancreatic cancer, the diagnostic accuracy of US for pancreatic cancer ranged from 64 to 90% according to various authors.
14,15,23,39) Fitzgerald et al.
15) reported that the diagnostic accuracy of US for pancreatic cancer was 67%, false negative 33%, and false positive 28%. Malagelad et al.
16) reported that sensitivity of US for pancreatic disease was 74%, specificity 84%, positive predictability 78% and negative predictability 79%. The diagnostic accuracy of CT for pancreatic cancer, was reported to be from 58.5% to 90%.
15,39,42ŌĆō44) Fitzgerald et al.
15) reported that the diagnostic accuracy of CT for pancreatic cancer was 94% with 60% false negative and 40% false positive. In our study, comparison of diagnostic accuracy among ERCP, US and CT could not be obtained because US and CT were not performed in all cause, and we thought that ERCP would be more sensitive than US and CT in evaluating for pancreatic cancer.
The location of pancreatic cancer was reported by Gleen and Thorbjarnarson
45) to be the head in 65%, the body in 42.8%, and the tail in 3. 8%. Gudjonsson et al.
4) reported the head, in 51 cases, the head and body in 5 cases, the body in 7 cases, the body and tail in 11 cases, the tail in 2 cases, and diffuse in 8 cases of a total of 84 cases. Fukumoto et al, reported the head in 25 cases, the body and tail in 16 cases, and diffuse in 4 cases in 45 cases and Choi et al.
31) reported the head in 66.6%, the head and body in 6.7%, the body in 6.7% and diffuse in 20% of 15 cases. In our study, the head was the most common (45.8%), followed by the body (39.3%), the tail (9.3%) and the head and body (9.3%). Levison explained that the head portion was the most frequent site of pancreatic carcinoma and surgical exploration of head portion for cancer was more commonly done than cancer of the other portions of the pancreas because pancreatic head cancer was manifested earlier due to jaundice.
The characteristic findings of ERCP in pancreatic cancer were obstruction, stenosis, narrowing and abnormal branching of the main pancreatic duct, acinar defect of the pancreas, stenosis (irregular and/or shouldered), obstruction, encasement and indentation of the common bile duct.
Silvis et al.
35) reported the ERCP findings of 37 cases of pancreatic carcinoma; pancreatic dud obstruction, 16 cases; pancreatic duct stenosis, 14 cases; and common bile duct obstruction or stenosis, 7 cases. Rhormann et al.
34) reviewed 500 pancreatograms and reported that 50 patients were found to have incomplete opacification of the main pancreatic duct. They classified the pancreatic duct termination into 6 types: (1) blunt, (2) nonspecific or HI-defined, (3) tapered, (4) meniscus, (5) eccentric, irregular of destructive, and (6) square. He reported that the duct termination in the 15 patients with pancreatic neoplasm was tapered, destructive, irregular, or eccentric in 73% (this couldnŌĆÖt be found in benign diseases) and nonspecific, or blunt, in 27%. Freeny et al.
36) reviewed 40 ERCP examinations retrospectively, to evaluate the accuracy and reliability of ERCP in the diagnosis of pancreatic carcinoma. He reported that 11 cases were diagnosed correctly by ERCP, and irregular or rat-tailed pancreatic duct obstruction was found in 8 cases, pancreatic duct encasement (nodular, and eccentirc narrowing) in 3 cases, and common bile duct obstruction or encasement in 6 cases. They have formulated some working hypotheses that (1) unless ERCP findings satisfy the criteria of pancreatic carcinoma described above, it must be inadequate, (2) if one major duct shows equivocal findings of carcinoma, it is essential to visualize the adjacent ducts if carcinoma involving that duct is to be excluded, (3) if one major duct shows equivocal findings of carcinoma and the adjacent ducts are normal, the disease involving that duct is probably benign, and (4) if a duct shows unequivocal findings of carcinoma, even in the midst of ducts involved with benign disease, that duct is considered to be involved by carcinoma until proven otherwise. Freeny et al.
21) reported that in 23 cases of pancreatic carcinoma, a positive ERCP diagnosis of carcinoma was made in 21 patients (95%) in whom one or both ducts were opacified. The findings of the main pancreatic duct were obstruction in 15 cases, encasement in 1 case, field (acinar) defect in 1 case, excavated cavity in 1 case, and normal in 3 cases (common bileduct encased). The findings on the common bile duct were obstruction in 1 case, encasement in 8 cases, and normal in 2 cases. They also suggested that the differential diagnosis of an obstructed pancreatic duct included incomplete filling, pancreatitis, neoplasm, and trauma. Incomplete filling is distinguished from other obstruction by incomplete side branch filling and a subtle fading or feathering of the ductal terminus. Chronic pancreatitis may lead to ductal obstruction by fibrosis, intraductal calculi, abscess or pseudocyst, and proximal to the obstruction, the main duct and secondary side branch usually show characteristic changes of chronic pancreatitis: ectasia, beading, multiple focal stenosis, marginal irregularities and calculi. They stated, that the ŌĆ£double duct signŌĆØ was the most reliable indicator of pancreatic carcinoma, and this was found in 5 of 9 patients in whom both ducts were visualized.
However, Ralls et al.
37) reported that 29 of 41 pancreatitis patients showed abnormalities of the pancreatic duct, such as irregular rat-tailed stenosis (8 patients) and nodular or eccentric narrowing (21 patients). The double duct sign was found in 15 of 41 patients, and pancreatic carcinoma could be diagnosed accurately if signs of pancreatitis were not present, although ductal abnormality was seen. Pulmley et al.
38) reported that the double duct sign was found in 52 (30 patients were proved to have pancreatic malignancy and 22, benign pancreatic disease), of 1,180 patients studied by ERCP. He concluded that the double duct sign was not a specific finding for pancreatic carcinoma, and that the character of the stenosis or obstruction, the distance from the papilla to the common bile duct stenosis, and the distance between biductal lesions have been shown to aid in the differentiation of benign from malignant disease.
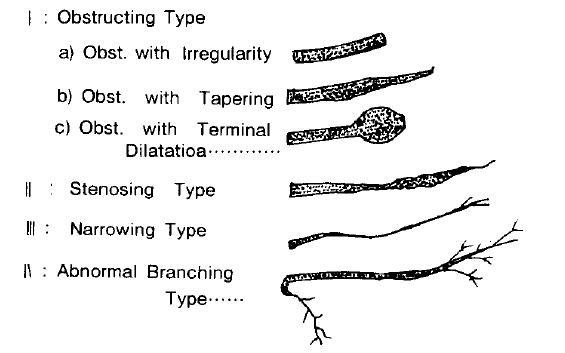
Fukumoto et al, classified the abnormal findings of the pancreatic duct of pancreatic carcinoma as Type I (obstruction) with Type Ia (obstruction with irregularity), Type Ib (obstruction with tapering), and Type Ic (obstruction with dilatation), Type II (stenosing), Type III (narrowing), and Type IV (abnormal branching). He reported that of the 31 cases of pancreatic carcinoma, Type I was the most common (20 cases; 64.9%) including Ia (14 cases), Ib (4 cases) and Ic (2 cases), followed by Type II (8 cases), Type III (2 cases), and Type IV (1 case). Choi
28) typed 37 cases of pancreatic carcinoma according to FukumotoŌĆÖs classification. Type I was 26 cases (70.3%), Type II, 10 cases (27.0%); Type III, 1 case (2.7%) and of the Type I, type Ia was 12 cases; Ib, 10 cases, and Ic, 4 cases. He stated that the ERCP findings of chronic pancreatitis were ectasia; beading, multiple focal stenosis, cyst formation, small cyst like ectasia, and calculi. Also, differentiation between carcinoma and chronic pancreatitis is very difficult occasionally, and that cases having both diseases may exist. In this study of 107 cases with abnormal pancreatic duct, the obstructive type was most common (65.4%), followed by the stenosing type (29%), the abnormal branching type (3.7%), and the narrowing type (1.9%) (
Table 4). FukumotoŌĆÖs classification according to the location of pancreatic cancer is as follows: The obstructing type was 59.2% (29 of 42 cases) in the head of the pancreas, 81.0% (34 of 42 cases) in the body 50.0% (5 of 10 cases) in the tail, and 33.3% (2 of 6 cases) in the head and body together. Of the 4 cases of the abnormal branching type, 3 cases (75%) were in the tail, and 1 case (25.0%) in the head (
Table 5).
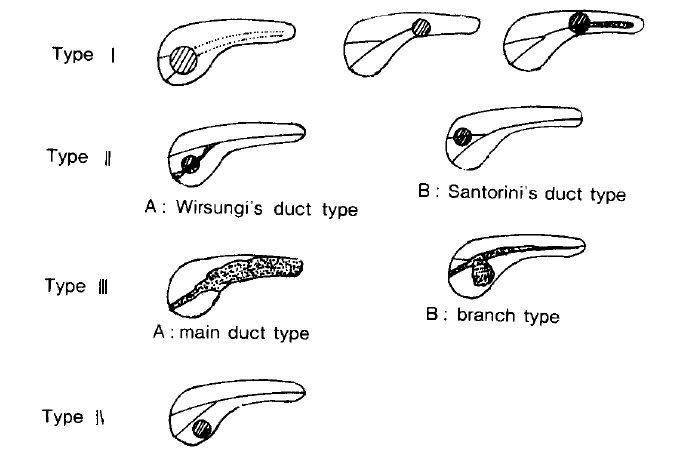
Takaki et al.
3) classified pancreatic cancer by ERCP findings as Type I (stenosis or obstruction of the main pancreatic duct and poststenotic dilatation), Type II (stenosis or obstruction of WirsungŌĆÖs or SantoriniŌĆÖs duct without poststenotic dilatation), Type III (ductal dilatation) and Type IV (abnormal pancreatic ducts). He reported that of 103 cases of pancreatic carcinoma, Type I was 86.4%; Type II, 5.8%; Type III, 5.8%; and Type IV, 1.9%, and that resectability was 34.8% in Type I, 83.8% in Type II, 100% in Type III, and 50% in Type IV.
In this study, of 111 cases with visualized pancreatic ducts, Type I was most frequent, 95 cases showing a rate of 85.6%; Type II was 1.8%; Type III, 9.0%; and Type IV, 3.6%. All 4 cases of Type IV with normal pancreatic ducts were diagnosed as carcinoma of uncinate process (2 cases) and tail (2 cases) by CT and US. Of the 122 cases with pancreatic cancer, common bile ducts were visualized in 55 cases and showed abnormalities in 28 cases, such as stenosis (42.9%), indentation (32.1%), and obstruction (25.0%).
Recently, various morphologic tests such as ERCP, US, angiography, and CT have been widely used for the diagnosis of pancreatic cancer, but early diagnosis of pancreatic cancer is still not easy. Several cases with small pancreatic cancer (below 2 cm in diameter) have been reported sporadically
25,26) but quite small in number. ERCP is a useful method for the diagnosis of pancreatic cancer, and also helpful in the prediction of resectability of caner, especially small cancer.






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


