 |
 |
| Korean J Intern Med > Volume 1(2); 1986 > Article |
|
Abstract
125I-insulin binding to erythrocytes was measured to evaluate the role of insulin receptors in the mechanism of insulin resistance in partients with chronic liver disease (CLD). Patients with CLD were divided into two groups by the fasting plasma glucose level (FBS) and the glucose disappearance rate (k-glucose) during intravenous glucose tolerance tests: one with normal FBS and normal k-glucose (group I), and the other with higher FBS and lower k-glucose (group II). Insulin receptor bindings were reduced in group I patients compared with those of normal controls, but were not reduced in group II patients. Fasting insulin levels were significantly higher in group I patients than those in normal subjects, and down-regulation by hyperinsulinemia might be the cause of reduced insulin receptor bindings in these patients. However down-regulation of insulin receptors was not associated with glucose intolerance. So it is suggested that insulin resistance in CLD is not due to a primary defect of insulin receptors in peripheral tissues, and down-regulation of insulin receptors is an epiphenomenon of hyperinsulinemia.
Insulin resistance and abnormal glucose tolerance are frequently observed in chronic liver disease (CLD), but the precise mechanism leading to insulin resistance still remains unclear1ŌĆō3). In an attempt to explain the mechanism of insulin resistance in these patients, several investigators have studied insulin receptor bindings in patients with CLD4ŌĆō7). However, the results are varied, and there remain controversies about the insulin receptor status in CLD.
Some studies have shown that insulin receptor bindings were reduced in CLD which suggested an insulin receptor defect as a primary cause of insulin resistance4ŌĆō5). However, other studies reported that insulin receptor bindings were normal6), or even increased7) in patients with CLD, thus raising doubt about the above explanation.
The present study was performed to elucidate this controversy by observing the insulin receptor status of erythrocytes in patients with CLD. Although it is not clear whether erythrocytes reflect the insulin receptor status in more relevant tissues such as liver and muscle, it is generally agreed that insulin bindings to erythrocytes and monocytes change in a parallel direction in many situations8).
The studies were carried out in eleven patients with CLD and in ten healthy subjects.
The patient group was composed of nine men and two women (age distribution; 25ŌĆō64 years of age, mean; 45). Eight patients had liver cirrhosis and three had chronic active hepatitis. They did not take any medications that might influence glucose intolerance, and evidence of recent gastrointestinal bleeding was absent. Serum creatinine, urea nitrogen and electrolytes were normal.
Control group was composed of 10 healthy men (age distribution; 33ŌĆō60 years of age, mean; 43). who showed no evidence of hepatobiliary, renal or endocrine disturbances. None had a family history of diabetes mellitus.
Intravenous glucose tolerance test (IVGTT) was performed after 25g of glucose was infused into an antecubital vein. Patients with CLD were divided into two groups by fasting plasma glucose levels (FBS) and the results of IVGTT. Group I was composed of 6 patients with FBS less than 100 mg/dl and normal glucose disappearance rate (k-glucose), and group II was 5 patients with higher FBS (FBS>100 mg/dl) and lower k-glucose values (Table 1).
Glucose disappearance rate (k-glucose) was calculated according to the formula of Amatuzio et al.19) as follow;
where C(t) is the glucose excess in mg/dl at any time t, and C(o) is the glucose excess above basal value in mg/dl at time zero.
The reticulocyte count in peripheral blood was performed in all of the samples. Those with over 1% reticulocytes were discarded as reticulocytes were known to have insulin receptors of more than tenfold compared with those of more matured red cells9).
Insulin binding to erythrocytes was studied in duplicate according to the method of Gambhir with some minor modifications as previously described10ŌĆō11).
Twenty milliliters of overnight fasting blood was drawn into a heparinized syringe and the erythrocyte cell layer was separated by Ficoll-Hypaque gradient centrifugation method. The cell suspension, trace amounts of radioactive insulin [125I-A14-monoiodinated human insulin with specific activity of about 2000 Ci/mmole (Amersham Co., England)]12), and various concentrations (0.02 to 105 ng/ml) of unlabeled insulin were incubated at 15┬░C for 3 and 1/2 hours. The bound form of insulin was separated and the radioactivity was counted in a gamma counter.
The nonspecific binding was defined as the radioactivity in the presence of 105 ng/ml of unlabeled insulin. The nonspecific binding was subtracted from the total binding and the results were expressed as specific 125I-insulin binding to erythrocytes of a concentration of 4.0├Ś109 cells/ml.
The results of the binding studies were presented as; (1) the specific binding of 125I-insulin in the absence of competing unlabeled insulin, % (B/T)0, (2) the specific cell bound fraction plotted as a function of total insulin concentrations (competition curve), and (3) The ratio of the cell-bound insulin to bee insulin plotted as a function of cell-bound insulin (Scatchard plots)13). In the Scatchard plots, the highest insulin concentrations used was 100 ng/ml14). The binding data were analyzed on the assumption that the curvature of the Scatchard plots reflects the presence of two populations of insulin binding sites (high affinity receptors and low affinity receptors)15).
Data in tables are given as mean┬▒S.D., and data in figures as mean┬▒S.E.M.. The statistical analysis of the data was performed using the unpaired t-test.
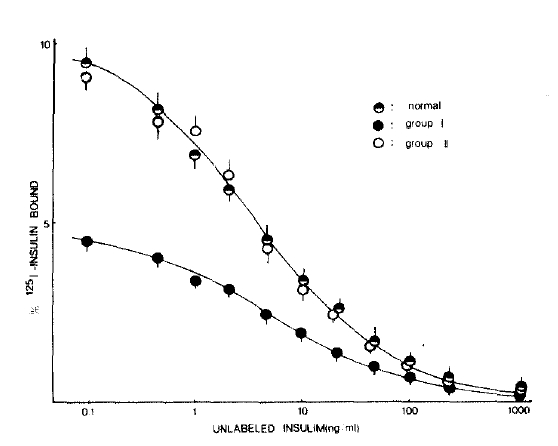
Fig. 1 shows the mean competition curves for 125I-insulin binding to erythrocytes in three study groups. The percent specific binding in the absence of unlabeled insulin was significantly lower in the group I patients compared with that of the normal subjects. However, 125I-insulin binding to erythrocytes in group II patients was very similar to that of the normal subjects.
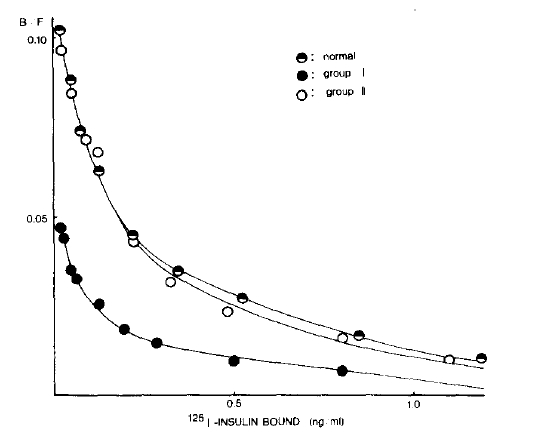
The Scatchard plots of the mean values at each concentration of the bound insulin in three groups are shown in Fig. 2. Apparent receptor numbers of both high and low affinity receptors tended to be lower in the group I patients than those in normal controls, but the statistical differences of these indices between two groups (group I and normal controls) were not significant probably due to the small size of the samples (Table 2). Scatchard plot in group II patients were almost identical to that of normal subjects.
There seem to be at least two subgroups of patients in CLD: one with normal FBS, normal k-glucose and brisk insulin response to intravenous glucose loading (group I), and the other with higher FBS, lower k-glucose and blunted acute insulin response to glucose loading (group II)16)(Table 1).
Present study clearly demonstrates that erythrocyte insulin receptor binding is also different between these two groups of patients. Insulin binding to erythrocytes was reduced in group I patients, but was not reduced in group II patients. This was quite surprising since if the primary cause of insulin resistance was the defect of insulin receptors in peripheral tissues, those patients with reduced insulin receptor binding would have more abnormal glucose tolerance than those with normal insulin receptor binding. This was not true in the present study, and it was suggested that insulin resistance in CLD might not be due to a primary defect of insulin receptors.
Reduced insulin receptor binding is frequently associated with many states characterized by insulin resistance and hyperinsulinemia, such as obesity, type II diabetes mellitus and acromegaly17). CLD is another state frequently associated with glucose intolerance, insulin resistance and hyperinsulinemia, and several investigators have reported that insulin receptor bindings to peripheral tissues are reduced in the patients with CLD. It has been believed that down-regulation of insulin receptor by hyperinsulinemia is the cause of insulin resistance in CLD4,5).
However, contradictory results have also been reported. Proietto et al6), have shown that insulin receptor binding was not altered in the patients with CLD, Okada7) reported that insulin binding was rather increased in the patients with CLD, and that the degree of increase was more marked in the patients who also had diabetes mellitus. The cause of the discrepancy is not clear. The result of the present study suggests that the discrepancy might be due to the differences in the study populations.
In the present study, fasting insulin levels in group I patients were significantly higher than those in normal subjects (Table 1.), and down-regulation by hyperinsulinemia might be the cause of reduced insulin receptor binding in these patients (Table 2.). However, down-regulation of insulin receptor was not associated with glucose intolerance (group I), and erythrocyte insulin receptor binding was almost normal in the patients with glucose intolerance (group II).
The biological significance of this phenomenon is not clear at present, and indeed it is not clear whether the observed changes in insulin binding to erythrocytes in CLD correlate with the changes in insulin binding to insulin sensitive tissues. Some investigators still believe that reduced insulin receptors in hepatic tissues may be the primary cause of insulin resistance in patients with CLD18).
However, no such direct evidence has been demonstrated until now, and at least erythrocyte insulin receptors are concerned, down-regulation of insulin receptors seems to be an epiphenomenon of hyperinsulinemia and might not be very important in the genesis of insulin resistance in these patients.
Fig.┬Ā1.
125I-insulin binding curves to erythrocytes in normal subjects, group I and group II patients with chronic liver disease.
Table┬Ā1.
Summary of the Glucose and Insulin Data in Fasting State and during IVGTT in Normal Subjects, Group I and Group II Patients with CLD
Table┬Ā2.
Insulin Binding Parameters in Normal Subjects, Group I and Group II Patients with CLD
REFERENCES
1. Conn HO, Schreiber W, Elkington SG, Johnson TR. Cirrhosis and diabetes. Am J Dig Dis 14:837. 1969.


3. Kingston ME, Ali MA, Atiyeh M, Donnelly RT. Diabetes mellitus in chronic active hepatitis and cirrhosis. Gastroenterology 87:688. 1984.


4. Teng CS, Ho PWM, Yeung RTT. Down-regulation of insulin receptors in postnecrotic cirrhosis of liver. J Clin Endocrinol Metab 55:524. 1982.


5. Blei AT, Robbins DC, Drobny E, Baumann G, Rubenstein AT. Insulin resistance and insulin receptors in hepatic cirrhosis. Gastroenterology 83:1191. 1982.


6. Proietto J, Nankervis A, Aitken P. Insulin resistance in cirrhosis: Evidence for a postreceptor defect. Clin Endocrinol 21:677. 1984.

7. Okada Y. Increased insulin binding to erythrocytes in chronic liver disease. Acta Med Okayama 35:155. 1981.

8. Wachslicht-Rodbard H, Gross HA, Rodbard D. Increased insulin binding to erythrocytes in anorexia nervosa: Restoration to normal with refeeding. N Engl J Med 300:882. 1979.


9. Eng J, Lee L, Yalow RS. Influence of the age of erythrocytes on their insulin receptors. Diabetes 29:164. 1980.


10. Gambhir KK, Archer JA, Carter L. Insulin radioreceptor assay for human erythrocytes. Clin Chem 23:1590. 1977.


11. Lee KU, Kim SY, Lee HK, Min HK. The effect of glucocorticoid on 125I-insulin binding to human erythrocytes: Possible postreceptor modulation of receptor binding. Diabetes Research Clin Practice 1:211. 1985.

12. Linde S, Hansen B, Sonne O, Holst JJ, Gliemann J. Tyrosine A (125I)-monoiodoinsulin preparation, biologic properties, and longterm stability. Diabetes 30:1. 1981.

13. Scatchard G. The attraction of proteins for small molecules and ions. Ann N Y Acad Sci 51:660. 1949.

14. Yasuda K, Kitabchi A. Decreased insulin binding of human erythrocytes after dexamethasone or prednisone ingestion. Diabetes 29:811. 1981.

15. Pederson O, Hyllund E, Lindskov HO. Circadian profiles of insulin receptors in insulin-dependent diabetics in usual and poor metabolic control. Am J Physiol 242:127. 1982.

16. Yoon KW, Woo EJ, Min BG, Rhee BD, Kim SY, Lee HK, Kim CY, Min HK. A Study on the mechanism of glucose intolerance in chronic liver disease Analysis of hepatic glucose balance by a computer simulation method during an intravenous glucose tolerance test. J Korean Diabetes Association 10:41. 1986.
17. Olefsky JM, Reaven GM. Decreased insulin binding to lymphocytes from diabetic subjects. J Clin Invest 54:1323. 1974.



18. Alberti KGMM, Johnston DG. Carbohydrate metabolism in liver disease. In: Wright R, Alberti KGMM, Karran S, Millward-Sadler GH, eds. Liver and Biliary Disease. 44. Saunders, London-Philadelphia-Toronto: 1979.
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 8,858 View
- 31 Download
- Related articles
-
Albumin for End-Stage Liver Disease2012 March;27(1)
Profiles of Serum Bile Acids in Live Diseases1986 January;1(1)
A Case of Glomerulonephritis in Association with Pyogenic Liver Abscess2001 September;16(3)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


