INTRODUCTION
Herpes simplex virus (HSV) infection of the respiratory tract was first reported in 1949 by Morgan, who cultured HSV from the lung tissue of the patient with severe burns and atypical pneumonia.1) Since then, it has been well known that HSV can cause fatal respiratory infection in patients with burns,2ŌĆō4) organ transplantation, malignancy, immunosuppressive therapy,5ŌĆō9) prolonged intubation,3,4,10) and alcoholic liver disease.11)
Recently, several cases of localized infection in the respiratory tract of the patient with normal resistance have been reported, in some of them recovery occurred spontaneously.12,13)
We, also, have seen a localized HSV tracheobronchitis in a patient with chronic obstuctive lung disease, which was confirmed by the cytologic changes of the bronchoscopic biopsy and brushing, and a positive culture of the bronchial washing. In Korea, there has been no report of a case of proven HSV infection confined to the lower respiratory tract.
CASE
A 78-year-old man was admitted with severe dyspnea and in a state of altered consciousness. He had suffered from cough, sputum, and exertional dyspnea for the previous 8 years. About 8 days before admission, respiratory symptoms had become worse with fever and chills, and treatment at a local clinic had failed to bring about any improvement. Two day before admission he had become drowsy.
Twenty-five years previously he had been treated with anti-tuberculous medication irregularly for 3 years and he had a history of smoking 60 pack year.
On physical examination, the patient appeared to be severely emaciated, cyanotic, and dyspneic. He was drowsy. His blood pressure was 120/80mmHg, pulse rate 132/minute, respiration rate 35/minute, and temperature 36.5┬░C. The anteroposterior diameter of the thorax was markedly increased and rhonchi and crackles were heard on both lower lung fields. The other parts were normal and there was no evidence of herpetic lesion on either the lips or oropharynx.
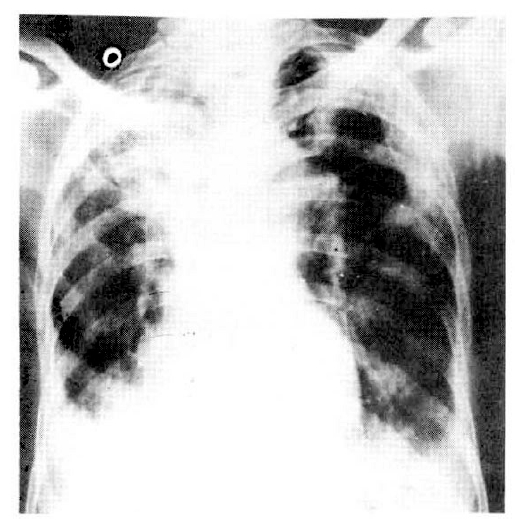
Hemoglobin was 12.1gm%, and WBC count was 28.000/mm3 with 90% neutrophils. An ECG revealed sinus tachycardia and left ventricular hypertrophy. Blood chemistry was normal except for mild hypoalbuminemia (2.7gm%). Arterial blood gas analysis on room air showed PO2 of 30mmHg, and PCO2 of 48mmHg. With 2L/min oxygen, PO2 was 50mmHg and PCO2 was 56 mmHg. Chest X-ray showed right upper lobe collapse and bilateral lower lung infiltration (Fig. 1).
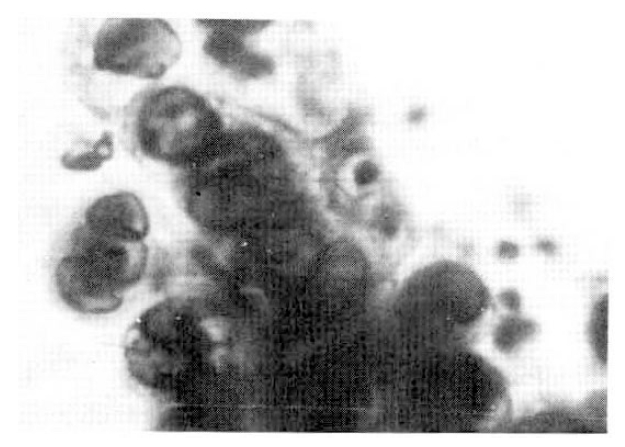
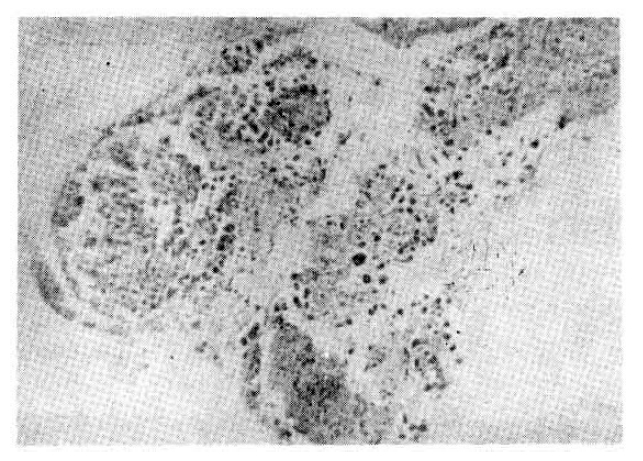
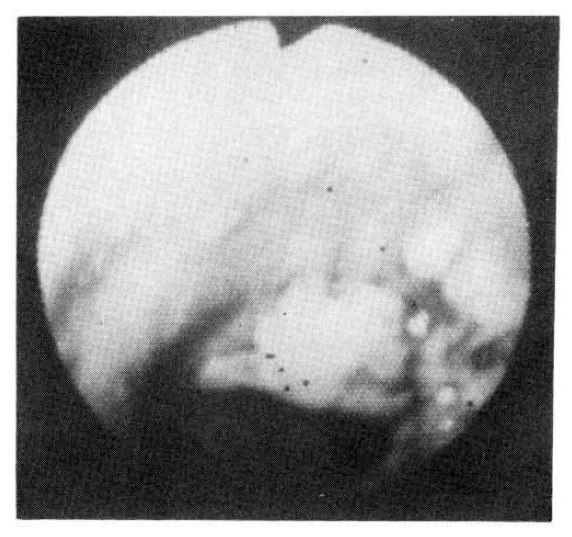
Six sputum smears for AFB were all negative. The patient improved with 2L/minute O2 inhalation, antibiotics (cephalosporin and gentamicin), brochodilatiors, and chest physiotherapy. On the 6th hospital day, fiberotic bronchoscopy was performed for the investigation of the lesion of the right upper lung. It revealed a localized area of swelling and hyperemia on the right lateral wall of the lower trachea, extending to the proximal part of right upper bronchus. No definite blister or ulceration was found, but several discrete nodules were noticecd (Fig. 2). At the cytologic study of broncheal brushing and washing, typical findings of HSV infection were observed : nuclei showed a ground-glass appearance with small, clear vacuoles, granular distribution of chromatin along the nuclear membrane, typical intra-nuclear eosinophilic inclusion bodies, and many, multinucleated ginat cells (Fig. 3). Bronchoscopic biopsy revealed severe necrotic inflammation with squamous metaplasia, and the cytologic changes typical of HSV, which were mentioned earlier (Fig. 4). Three days later a repeated bronchoscopy was done to obtain material for culture, and this time, whitish pseudomembrane was found to cover the inflamed area (Fig. 5). Viral culture by the inoculation of the bronchial washing into Vero Cells (monkey kidney cells) with medium-199 at the Korean National Institute of Health resulted in the growth of HSV type I.
Because on the 12th hospital day, the patientŌĆÖs condition began to get worse, with increasing sputum production and dyspnea, acyclovir was started. On the 3rd day of acyclovir therapy, the patient complained of a sudden onset of severe chest pain, and collapsed. An ECG revealed extensive anterior wall myocardial infarction and he died several hours later.
Blood drawn before death revealed 480 units of S-GOT, 380 units of CPK, and 2,651 units of LDH.
DISCUSSION
HSV is common pathogen invading the oropharyngeal mucosa, the skin, the genitalia, and the gastrointestinal tract, but respiratory tract involvement is relatively rare. After the first description in 1949 by Morgan,1) increasing numbers of cases have been reported.
Most of the respiratory infections were fatal in an immune-compromised host,5ŌĆō9) in severe burns or in prolonged intubation with adult respiratory distress syndrome,16) and the diagnosis was made only by autopsy.1,3,4) However, recently, more cases with an antemortem diagnosis5,9,12,13,16ŌĆō20) or localized infection which has healed spontaneously have been reported.
For the HSV infection, both humoral and cellular immunity are present, but like other viral infections, cellular immune response is thought to be much more important.21ŌĆō23) Arvin reported a defect in lymphocyte transformation reaction on the challenge of HSV in the patient with lymphoma and recurrent HSV infection.20) Wilton observed the impaired release of the migration inhibition factor (MIF) from macrophage and lymphocyte cytotoxicity to HSV infection.22) Since OŌĆÖReilly also found a defective production of interferon and leucocytic release of MIF, an impaired cellular immune response has been suggested as a predisposition for recurrent HSV infection. In addition, Drew discovered the importance of alveolar macrophages in the defense against HSV.24) Therefore, a defect in the local immune response as well as impired systemic immunity can be a predisposing factor for respiratory HSV infection.
Because HSV usually invades squamous epithelium, the respiratory tract is not a frequent site of infection. However, after the squamous metaplasia became induced by smoking, smoke inhalation during burns, or prolonged intubation. HSV infection became more frequent. In fact, most of the reported HSV infections have occurred in the areas of squamous metaplasia.19,25) Rarely, it can occur in the elderly patient without underlying disease,4,13,25,26) or even in the younger normal person.12,13) Our patient was a heavy smoking, elderly patient with chronic obstructive pulmonary desease, and squamous metaplasia was found on bronchoscopic biopsy.
HSV is relatively common inhabitant of the human salivary gland27) and in 2.7ŌĆō11.5% of the patients with other respiratory disease, HSV has been cultured from the sputum or throat swab.28,29) Also, the fact that mucocutaneous HSV infection has been frequently found before, or at the same time of respiratory ilness suggests the contagious spread or inhalation of contaminated material as a route of respiratory infection. However, hematogenous dissemination or spread via the nerve pathway have also been suggested.30,31)
The diagnosis of HSV infection can be made by viral culture, typical cytologic changes, histological findings, and the direct immunofluorescent study of infected tissue. Viral culture is a very sensitive and rapid test but in respiratory infection, active infection cannot be differentiated from colonization. In one study viral culture of the oropharyngeal secretions was positive for HSV in 1ŌĆō5% of normal adults29) and 2.7ŌĆō11.5% of patients with respiratory disease of other kinds.28,29) In the patient with pharyngitis, up to 22% of the pharyngeal swabs were positive.28) Therefore, a positive culture only is not sufficient for making a diagnosis. On the contrary, typical cytopathologic or histopathologic changes are the evidences of active infection. The ground-glass appearance of nucleus with multiple small vacuoles, the eosinophilic intranuclear inclusion bodies, and the multinucleated ginat cells, which were seen in our case, are characteristic findings in HSV infections. Other viral infections can cause similar changes, but in cytomegalovirus infection, both intranuclear and intracytoplasmic inclusion bodies are present. The fact that inclusion bodies are usually basophilic and the huge size of an infected cell can make the diagnosis clear.32,33) Adenovirus can cause multiple inclusion bodies only in the nucleus, but multi-nucleated giant cells are usually absent.32,33) In respiratory synctytial viral infection, inclusion bodies are all intracytoplasmic.32,34)
In view of this, Ramsey proposed the following findings as criteria for the diagnosis of HSV pneumonia30) : parenchymal infiltration on the chest X-ray, positive viral culture from the lung tissue, and hemorrhagic or ulcerative inflammation with typical cytopathologic changes in the lung tissue from which the culture was taken.
Our case had definite HSV tracheobronchitis on the basis of RamseyŌĆÖs criteria. However, it is not likely that he had HSV pneumonia, too, because the right upper lung density on chest the X-ray was old atelectasis rather than infiltration, and the distal part of the bronchial mucosa of the right upper lobe was normal on the bronchoscopy.
The bronchoscopic appearance of HSV infection is not charactersistic. Acute inflammatory change, with or without blisters, ulceration, or pseudomembrane has been described.13,16) In our case, pseudomembrane on top of acute inflammation was found on the second bronchoscopy.
The treatment of HSV is still unsatisfactory,5,13) but after the administration of adenosine arabinoside (Ara-A), the mortality and morbidity has been decreased.14) Ara-A has been reported to be effective for severe mucocutaneous HSV infection.35 Recently, acyclovir which is more potent and has fewer side effects, has been used with good results.15,20,36)




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





