INTRODUCTION
Polyarteritis nodosa is a vasculitis characterized by fibrinoid necrosis of the medium-sized arteries. The clinical picture depends on which organs are made ischemic by this pathological process. Ureteral obstruction is a rare manifestation of polyarteritis nodosa and thought to result from vasculitis of periureteral vessels.
We report a case of bilateral ureteral obstruction in polyarteritis nodose that resolved completely with medical treatment.
CASE REPORT
A 56-year-old Korean woman presented with a 3-month history of fever, weight loss and generalized myalgia. From 6 weeks before hospitalization, the myalgia had worsened and progressive proximal muscle weakness developed.
At hospitalization, the temperature and blood pressure were 38.6┬░C and 110/70. Physical examination showed an emaciated woman in chronic distress. There was severe muscle tenderness over lower and upper extremities with decreased proximal muscle power of grade III. On abdomina examination, moderate tenderness was noted over right and left upper quadrants without rebound tenderness. Otherwise, the physical examination was unremarkable. Urinalysis was normal. Sedimentation rate was 102 mm/hr, leukocyte count 16,000/mm3 and hematocrit 30 percent (normal 36 to 47). GOT and GPT were elevated up to 110 IU/L (normal<40 IU/L), 72 IU/L (normal<40 IU/L), alkaline phosphatase 2054 IU/L (normal<220 IU/L). HBsAg was negative. Rheumatoid factor was 80 IU/ml (normal<25 IU/ml). P-ANCA was positive with the titer of 1 : 80. The results of the following studies were normal or negative: electrolytes, creatine kinase, LDH, serum creatinine, antinuclear antibodies and anti-double strand DNA titters.
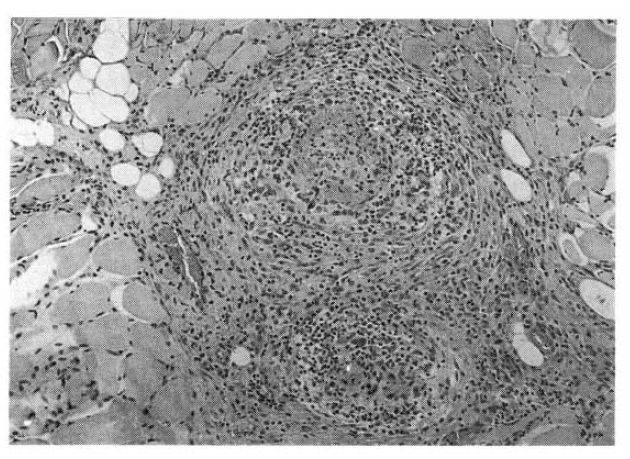
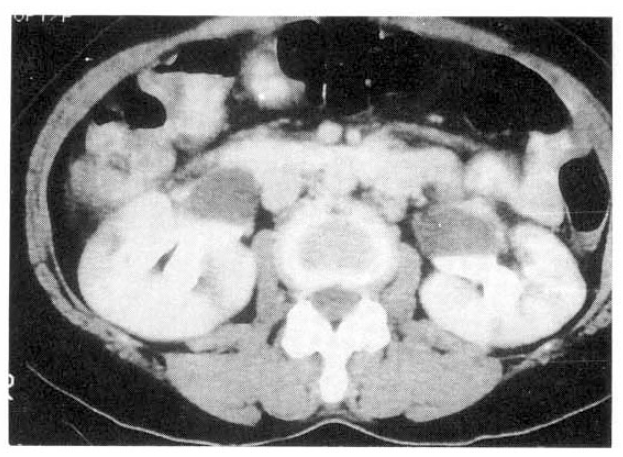
The patient underwent muscle biopsy from left quadriceps femoris for evaluation of the progressive myalgia and weakness. Findings were typical of necrotizing vasculitis (Fig. 1a). Renal angiography showed multiple microaneurysms which were all consistent with the diagnosis of polyarteritis nodosa (Fig. 1b). The ultrasound of the abdomen revealed bilateral hydronephrosis with normal cortical thickness. The retrograde pyelogram (RGP), as well as excretory urography (IVP), revealed bilateral hydronephrosis to the level of the midureter without evidence of calculi or tumor. The computerized tomography (CT) of the abdomen and pelvis confirmed these findings (Fig. 2) and also showed foci of abnormal soft tissue adjacent to the areas of both ureteral narrowings without evidence of mass or lymphadenopathy.
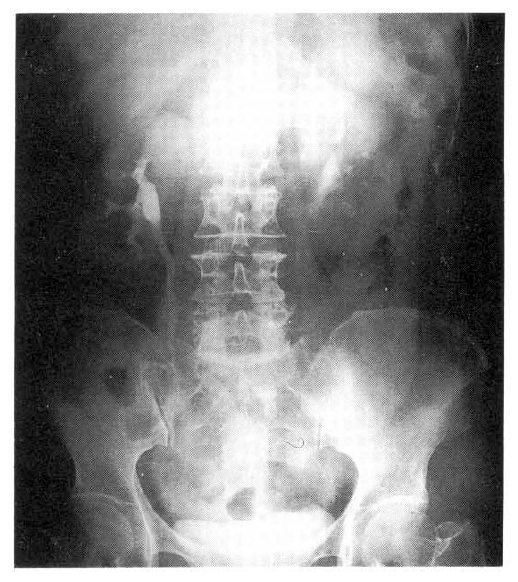
Oral prednisolone (1 mg/kg) was begun and indwelling ureteral stents were inserted to each ureter to relieve the obstructions that were thought relatively acute in onset based upon the maintained cortical thickness by ultrasound. At the time of ureteral stent insertion, ureteroscope was also performed to visualize the obstructed segment but there was no intraluminal abnormality around the narrowed portions. Although the main cause of obstructions was thought to exist outside of the narrowed ureters, given the periureteral soft tissue densities shown in abdominal CT, a blind mucosal biopsy from the narrowed segment was done and showed only fibrous tissue without any evidence of malignancy or vasculitis. After 4 weeks of prednisolone, follow-up ultrasound of the abdomen and excretory urography showed no evidence of hydronephrosis. The right ureteral stent was removed first and 10 days later another excretory urography was performed which showed again complete resolution of the obstructions. The other ureteral stent was removed the next day and a final excretory urography was done 2 months after the initiation of medical treatment and was normal (Fig. 3). Clinically, the patient improved with immediate defeverscence, decrease of constitutional symptoms, gradual improvement of myalgia and muscle weakness and normalization of liver function tests and sedimentation rate down to 17 mm/hr. At 24 monthsŌĆÖ follow-up, the patient is off steroid after slow tapering with no evidence of recurrent ureteral obstruction confirmed by abdominal ultrasound.
DISCUSSION
Ureteral obstruction in polyarteritis nodosa is a very rare condition. In our literature review, there have been 16 cases reported on ureteral stenosis in polyarteritis nodosa since the first description in 1948 by Fisher and Howard1ŌĆō9). The presented case is unique in that ureteral obstruction thought due to polyarteritis nodosa occurred bilaterally, synchronously and resolved completely with medical treatment alone. All reported cases of ureteral involvement by polyarteritis nodosa had to be operated and therefore all could be confirmed by tissue biopsies. In our case, the diagnosis of polyarteritis nodosa was done rapidly by the typical muscle histology, although the cause of the bilateral hydronephrosis was an enigma for several days until we excluded other possible causes of ureter obstructions. No evidence of calculi or mass could be found throughout computerized tomography of abdomen and pelvis and excretory urograms. The patient reported on by Baskin and associates7) presented similarly with unilateral ureter obstruction in which the pelvic CT showed soft tissue density adjacent to the narrowed ureter, and later confirmed by operational biopsy as necrotizing vasculitis with extensive fibrinoid change.
The mechanism of vasculitis in polyarteritis nodosa may be secondary to immune complex deposition along the walls of medium and small arteries. Such a process promotes infiltration with polymorphic leukocytes and liberation of necrotizing enzymes. This leads to thrombosis, tissue ischemia and fibrosis, and ultimately tissue scarring4). We may hypothesize that the vasculitic process in polyarteritis nodosa resulted in ureteral vascular ischemia that, in turn, caused bilateral, synchronous periureteral fibrosis leading to ureteral stenosis in this case, which is supported by the excellent response to the steroid, exclusion of other causes of ureteral obstruction and very similar in presentation to the previous case confirmed by operation as necrotizing vasculitis. In our case, operative intervention was reserved for failure with medical treatment and so we could not prove whether or not the obstructing lesions were ureteral lesion or periureteral lesion, such as retroperitoneal fibrosis reported in polyarteritis nodosa.
In treatment, there is no specific thearpy for the ureteral stenosis if the treatment of underlying polyarteritis nodosa would affect the vasculitic process around the ureter. Surgical intervention would be performed in case medical treatment alone does not resolve the stenosis. A review of the literature reveals 5 cases of ureteral obstruction caused by the vasculitis of WegenerŌĆÖs granulomatosis10ŌĆō14). Of these cases, 3 were managed with surgical intervention followed by medical therapy, and 2 with medical therapy alone. All cases showed improvement of the obstructive uropathic condition. There is also a couple of cases of ureteral obstruction in systemic lupus erythematosus, in which obstruction was relieved with corticosteroid therapy7,15). In our case, there was no abnormality in urinalysis or renal function except bilateral flank tenderness. So, the main reason for the excellent response to steroid therapy in our case of ureteral obstruction might be relatively early recognition of the hydronephrosis, accordingly with less scar changes around the ureters. Our case illustrates the value of steroid treatment in relieving ureteral stenosis in polyarteritis nodosa sparing surgical intervention.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





