 |
 |
| Korean J Intern Med > Volume 2(1); 1987 > Article |
|
Abstract
The coexistence of nephrotic syndrome and renal vein thrombosis has been of medical interest since RayerŌĆÖs description in 1840. Renal vein thrombosis has been underdiagnosed because of its variable clinical and radiological findings but it becomes a more frequently recognizable clinical entity since diagnosis can be easily established by modern angiographic techniques.
Generally it has been believed that renal vein thrombosis may cause nephrotic syndrome. But recent articles strongly suggest that renal vein thrombosis is a complication of the nephrotic syndrome rather than a cause. We report three cases of nephrotic syndrome associated with renal vein thrombosis.
Since the association of nephrotic syndrome and renal vein thrombosis was first described by Rayer in 18401), several reviews of this association have been published2ŌĆō6). With the developement of more advanced radiographic techniques, selective catheterization and more understanding on renal vein thrombosis in nephrotic syndrome, the diagnosis or renal vein thrombosis and studies of its cause and effect have been made with increasing frequency7ŌĆō13).
We report three cases of renal vein thrombosis associated with nephrotic syndrome which were confirmed by abdominal computed tomographic scan, vena cavography, renal angiography and renal biopsy.
A 15-year-old boy was hospitalized for evaluation of left side pain of 2 days duration and intermittent edema for the past 2 years.
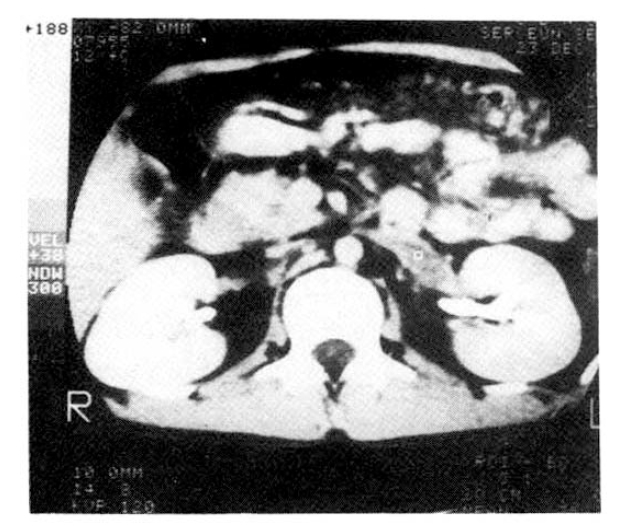
He was diagnosed as having membranoproliferative glomerulonephritis when he was 13. There-after he was treated with Cytoxan and Prednisolone for 3 months. Admission physical examination disclosed severe edema on lower extremities and scrotum, hemorrhoid, ascites and moderate knocking tenderness on the left flank. A urinalysis showed 3+ protein, 1+ sugar, 3ŌĆō4 WBCs and 10ŌĆō20 RBCs per high power field. Urinary protein excretion showed fluctuation from 7.0 gm/24 hr to 20.3 gm/24 hr. Laboratory data included albumin 1.2 gm%, globulin 1.3 gm%, glucose 90 mg%, BUN 30 mg%, cratinine 1.2 mg% cholesterol 615 mg% and triglyceride 431 mg%. Abdominal computed tomographic scanning showed a hypodense lesion in the left renal vein (Fig. 1), which was confirmed by renal venography. The patient was started on a regimen of Dipyridamol and Aspirin. After 1 week, the pain was being controlled with an improved general condition. The patient was continued on the regimen.
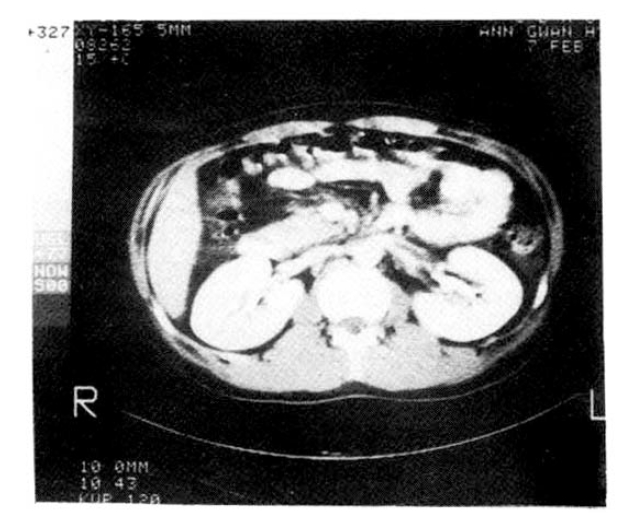
A 27-year-old man was hospitalized for evaluation of left flank pain of 1 week duration. He was diagnosed as having nephrotic syndrome 6 months ago and treated with Prednisolone. The admission physical examination disclosed generalized edema especially in lower extremities. A urinalysis showed 3+ protein, 4ŌĆō6 RBCs and 7ŌĆō15 WBCs per high-power field. Urinary protein excretion showed a fluctuation from 3.5 gm%/24 hr to 10.0 gm%/24 hr. Laboratory data showed albumin 1.9 gm%, globulin 1.6 gm%, cholesterol 480 mg% and triglyceride 375 mg%. Differential studies on urine specimens revealed protein 5.8 gm%/24 hr, osmolality 298 mosm/l, Na 62 mEq/l, K 32 mEq/l and CI 46 mEq/l from the left ureter and protein 5.6 gm%/24 hr, osmolality 361 mosm/l, Na 63 mEq/I, K 42 mEq/l and CI 70 mEq/l form the right ureter. Abdominal computed tomographic scanning showed a hypodense lesion in the left kideny and renal venography showed a thrombus in distal renal vein (Fig. 2). The patient was treated with heparin and anticoagulants. On this regimen the pain disappeared and the renal function remained normal.
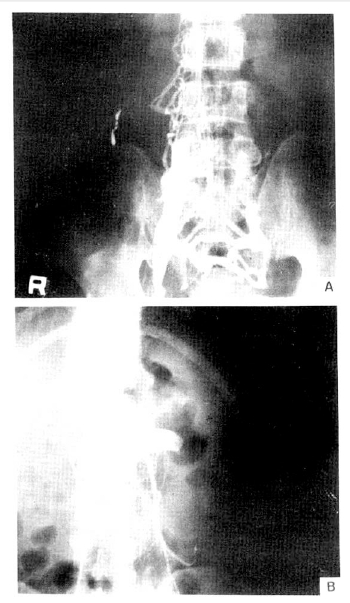
A-47-year-old man was hospitalized for evaluation of right flank pain of 3 days duration. He was otherwise asymptomatic and denied having trauma, edema or renal disease. A urinalysis showed 3+ protein, 1+ sugar, 7ŌĆō10 RBCs and 8ŌĆō10 WBCs per high-power field. Urinary protein excretion was 2.1ŌĆō3.2 gm/24 hr during admission. Laboratory data included albumin 3.9 gm%, globulin 3.3 gm%, glucose 104 mg%, cholesterol 322 mg% and triglyceride 117 mg%. A needle biopsy specimen of the kidney revealed membranous changes. Immunofluorescent study showed linear deposit of IgG. Abdominal computed tomographic scanning showed a large thrombus in inferior vena cava and dilated azygos vein. Inferior vena cavogram showed complete obstruction in iliac vein region and abundant collaterals (Fig. 3). The patient was treated with anticoagulants and continued to do well.
In 1840, Rayer1) decribed 7 cases of renal vein thrombosis, of which 2 had clinical features of the nephrotic syndrome. Since Derow2) described a case of chronic thrombosis of the renal vein and inferier vena cava with the clinical picture of nephrotic syndrome in 1939, there have been a number of reports of the association of renal vein thrombosis and nephrotic syndrome3ŌĆō6). It is generally agreed that the renal vein thrombosis is responsible for the development of the proteinuria of nephrotic syndrome and renal vein thrombosis is a rare but well-recognized cause of nephrotic syndrome3ŌĆō5,9,10,14). However several lines of evidence in recent articles have questioned such hypothesis and strongly suggested that renal vein thrombosis is a consequence of the nephrotic syndrome rather than a cause, judging by the increasing number of case reports and various animal experiments15ŌĆō23).
First, prospective long-term evaluation of nephrotic patients showed normal venogram and subsequent development of renal vein thrombosis18,19,24ŌĆō26). Second, nephrotic syndrome itself is associated with hypercoagulable state and the patients with the nephrotic syndrome exibit a high incidence of thrombotic phenomena in vessels other than the kidney itself, for examble inferior vena cava, pulmonary artery and mesenteric artery25,27). Third, selective renal function studies in unilateral renal vein thrombosis showed similar total protein excretion and protein selectivity for each kidney21,28). The findings of Case 2 were in agreement with such a concept. Fourth, renal vein obstruction induced in experimental animals caused mild proteinuria and pathologic change in the renal biopsy specimen3,23). Omae et al.29) found that excision of the intact contralateral kidney one week after partial occlusion of one renal vein was followed by development of a nephrotic syndrome with some thickening of glomerular basement membranes visible by light microscopy. Fisher et al.22) confirmed the development of nephrotic syndrome in rats. Although increased permeability of glomerular lamina densa was demonstrated in nephrotic kidneys, deposit or other overt membranous changes were lacking. Harris et al.14) reported thickened basement mambrane and deposit of r-globulin in glomeruli of dogs after total constriction of one renal vein and suggested that the damage to the affected kidney produced an autoimmune reaction in the other. Polenakovic et al.23) found that increased proteinuria did occur during the first 3 days after obstruction of a renal vein to 0.5 mm in rats. However he failed to find any difference from control animals in the amount of proteinuria and any evidence of membranous glomerulitis in renal biopsy specimen.
It is well-known that renal vein thrombosis occurs mainly in membranous glomerulopathy and less frequently in membranoproliferative glomerulonephritis. The incidence of renal vein thrombosis in the retrospective studies of membranous glomerulonephritis and membranoproliferative glomerulonephritis has been found to be 33% by Trew et al.30), 40% by Llach et al.24) and 50 % by Bennet et al.31) Other renal lesions that are associated with renal vein thrombosis are minimal change lesion, focal sclerosis13), rapidly progressive glomerulonephritis, malignancy25), and IgA nephropathy20). Systemic diseases involving kidney associated with renal vein thrombosis are lupus nephritis18), amyloidosis26), diabetic nephropathy, sickle cell anemia, polyarteritis nodosa33), malignant hypertension25), and paroxysmal nocturnal hemoglobulinuria32).
Although the pathogenetic machanism of renal vein thrombosis in nephrotic syndrome is not completely understood, it is established that the nephrotic syndrome is associated with a hypercoagulable state.
Patients with nephrotic syndrome have an increase in the number of platelets and in blood levels of fibrinogen, factor V, combined factors VIII and X, accelerated thromboplastin generation and increased activation of Hagemann factor26,27). The milieu is enhanced further by the urinary loss and depressed serum level of antithrombin III, so critical to the inhibition of several clotting proteases21). Poor fibrinolysis because of increased plasmin inhibitors and reduced blood flow because of decreased plasma volume and cariac output also has a role36). Furthermore the coagulation abnormalities returned to normal with the remission of nephrotic state26,35). In this situation, diruretics, corticosteroids and oral pills which may exert thrombogenic activity should be used with caution22,27,37).
The clinical features are determined by the balance between the acuteness of occlusion, extension of the thrombosis and development of collateral circulation33). Acute presentation of renal vein thrombosis is infrequent and is observed frequently in the younger patients with acute flank pain and marked costoverterbral angle tenderness. Chronic renal vein thrombosis is observed frequently and patients are usually older and relatively asymptomatic. Complaints will be of a chronic nature, that is, nausea, apathy, weakness and generalized edema, or they may be absent completely9,33).
Laboratory findings which may suggest renal vein thrombosis are variability in proteinuria and in creatinine clearance, hematuria, pyuria, glycosuria, and hyperchloremic acidosis. The variability in proteinuria and creatinine clearance also shown in our cases is probably due to varying degree of venous obstruction and recurrent thrombus formation in the renal vein.
Formation of collateral channels and their subsequent thrombus may be a second cause9). Our Case 3 was very unusual in that serum albumin was 3.9 gm% and urinary protein excretion was 2. 1ŌĆō3.2 gm/24hr. Considering the correlation between serum albumin level and antithrombin III by Kauffmann et al.34) and some animal experiments which showed proteinuria after renal vein obstruction14,22,29), we cannot rule out the possibility that it might be a beginning of development of nephrotic syndrome after longstanding renal vein thrombosis.
Renal vein thrombosis in nephrotic syndrome is thought to be underdiagnosed because it has variable clinical and radiological presentations and no local signs or symptoms at all in many instances24,26,33,38). The early diagnosis of renal vein thrombosis is very important because it is a treatable condition and radiologic evaluation is essential for it. An intravenous pyelogram, usually the initial investigation of choice, can suggest thrombosis but is insufficient for definitive diagnosis. It may reveal asymmetry in kidney size and function if thrombosis is unilateral and notching of the ureter due to dialated collaterals28,33). Renal arteriography with late films of the venous phase can be the first angiographic procedure because it can assess the renal parenchyma, blood flow and presence of collaterals without the risk of dislodging thrombi33,40). But the most definitive diagnostic modality is renal venography. Care must be exercised in its performance because of the risk of dislodging thrombi with resultant pulmonary embolism and in its interpretation because ŌĆ£streamingŌĆØ artifact made by the normally high rate of blood flow entering the vena cava from the renal vein may lead to an erroneous diagnosis.
Accurate diagnosis can be made if contrast material is visualized surrounding a filling defect in the renal vein10,42). Recently, Doppler ultrasound and abdominal computed tomographic scanning have been used as rapid and safe noninvasive diagnostic measures for visualizing thrombus in renal vein directly39,41,43). Abdominal computed tomographic scanning showed thrombi in all of our cases.
As with renal vein thrombosis, both medical and surgical treatment has been advocated. Although there have been a lot of reports of a favorable outcome after thrombectomy3,7,9,10), the treatment has shifted from a surgical to a medical focus during the last 20 years33,44). The objective of anticoagulant therapy is not only to minimize the occurance of pulmonary embolism but also to retard growth of a renal vein thrombus, thereby helping to preserve renal function and prevent the spread to the vena cava or to the other renal vein8,9,26,44,45). Once the diagnosis has been establish, hepatization followed by warfarin is the initial treatment of cloice33).
On the other hand, thrombectomy might be indicated in unrelenting pulmonary emboli and oliguria refractory to medical therapy from bilateral renal vein thrombosis or thrombosis of a solitary kidney33). Reports of other therapeutic measures have included the administration of Dextran46), Dipyridamol47), and the thrombolytic agents Urokinase and Streptokinase48ŌĆō50) but they need more extended experience.
REFERENCES
1. Rayer PFO. Traite des Maladies des Reins et des Alterations dela Secretions Urinaire. 2:590. Paris: JB Balliere Publishers, 1840.
2. Derow HA, Schlesinger JM, Savitz HA. Chronic Progressive Occlusion of the Inferior Vena Cava and the Renal and Portal Veins with the clinical Picture of the Nephrotic Syndrome: Report of A Case with Review of the Literature. Arch Intern Med 63:626. 1939.

3. Morris JF, Cinn HE, Thompson DD. Unilateral Renal Vein Thrombosis Associated with the Nephrotic Syndrome. Am J Med 34:867. 1963.

4. Sturgill BC, Rowe CT. Renal Vein Thrombosis and the Nephrotic Syndrome. Arch Intern Med 120:708. 1967.


5. Pollak V, Kark RM, Pirani CL, Muehrcke RC. Renal Vein Thrombosis and the Nephrotic Syndrome. Am J Med 21:496. 1956.


6. McCarthy LJ, Titus JL, Daugherty GW. Bilateral Renal Vein Thrombosis and the Nephrotic Syndrome in Adults. Ann Intern Med 58:837. 1963.


7. Duffy JL, Letteri J, Cinque T, Hsu PP, Molho L, Churg J. Renal vein Thrombosis and the Nephrotic Syndrome. Report of Two Cases with Successful Treatment of One. Am J Med 54:663. 1973.


8. Balabanian MB, Schnetzler DE, Kaloyanides GJ. Nephrotic Syndrome, Renal Vein Thrombosis and Renal Failure. Report of Case with Recovery of Renal Function, Loss of Proteinuria and Dissolution of Thrombus after Anticoagulant Therapy. Am J Med 54:768. 1973.


10. Janower ML. Nephrotic Syndrome secondary to Renal Vein Thrombosis. The Value of Inferior Vena Cavography. Am J Roentgen 131:843. 1978.

11. Kowal J, figur A, Hitzing WM. Renal Vein Thrombosis and the Nephrotic Syndrome with complete remission. J Mt Sinai Hosp 30:47. 1963.
12. Schwartz MM, Lewis FJ. Immunopathology of the Nephrotic Syndrome Associated with Renal Vein Thrombosis. Report of Two Cases and Brief Review of the Literature. Am J Med 54:528. 1973.


13. Mun HB, Ahn CR, Kwon IS, Kim SK. A Study on the Thrombotic complications in Nephrotic syndrome. Korean J Nephrology 2:31. 1983.
14. Harris JK, Ehrenfeld WK, Lee JC, Wylie EJ. Experimental Renal Vein Occlusion. Surg Gynec and Obst 126:55. 1968.
17. Luft FC, Walker PD, Hamburger RJ, Kleit SA. Thrombosis of the Renal Veins and Vena Cava Occurrence in Morbid Obsity. J Am Med Ass 234:1158. 1975.

18. Bradley WG Jr, Jacobs RP, Trew PA, Biava CG, Hopper J Jr. Renal Vein Thrombosis: Occurrence in Membranous Glomerulonephropathy and Lupus nephritis. Raiology 139:571. 1981.

19. Older RA, Miller MD, Tisher CC. Renal Vein Thrombosis, complication of Primary Renal Disease and the Nephrotic Syndrome. J Am Med Ass 240:1747. 1978.

20. McMorrow RG, Curtis JJ, Luke RG. Renal Vein Thrombosis in IgA Nephropathy. Arch Intern Med 140:700. 1980.


21. Kauffmann RH, de Graeff J, de La Raviere GB, Van Es La. Unilateral Renal Vein Thrombosis and Nephrotic Syndrome. Report of A Case with Portein Selectivity and Antithrombin III Clearance Studies. Am J Med 60:1048. 1976.


22. Fisher ER, Sharkey D, Parco V, Vuzevski V. Experimental Renal Vein Constriction. Its Relation to Renal Lesions Observed in Human Renal Vein Thrombosis and the Nephrotic Syndrome. Lab Invest 18:689. 1968.

23. Polenakovic M, Ganote CE, Potter EV, Jennings RB. Unilateral Renal Vein Occlusion in Rats. Nephron 40:91. 1985.


24. Llach F, Arieff Al, Massry SG. Renal Vein Thrombosis and Nephrotic Syndrome. A Prospective study of 36 Adult patients. Ann Intern Med 83:8. 1975.


25. Llach F, Koffler A, Finck E, Massry SG. On the Incidence of Renal Vein Thrombosis in the Nephrotic Syndrome. Arch Intern Med 137:33. 1977.

26. Llach F, Papper S, Massry SG. The Clinical Spectrum of Renal vein Thrombosis: Acute and Chronic. Am J Med 69:819. 1980.


27. Kendall AG, Lohmann RC, Dossetor JB. Nephrotic Syndrome A Hypercoagulable State. Arch Intern Med 127:1021. 1971.


28. Trygstad CW, McCabe E, Francyk WP, Crummy Ab. Renal Vein Thrombosis and the Nephrotic Syndrome: A Case Report with protein Selectivity Studies. J Ped 76:681. 1970.

29. Omae T, Masson GMC, Corcoron AC. Experimental Production of Nephrotic Syndrome Following Renal Vein Constriction in Rats. Subtotal Ligation of One Renal Vein and Contralateral nephrectomy. Proc Soc Exp Biol Med 97:821. 1958.


30. Trew PA, Biava CC, Jacobs RP. Renal Vein Thrombosis and Membranous Glomerulonephritis. In : From the Abstract of the seventh annual meeting of the American Society of Nephrology; Washinton DC. Nov 25ŌĆō26, 1974; p. 74.
31. Bennet WM. Renal Vein Thrombosis and Nephrotic Syndrome: Comments and correction. Ann Intern Med 83:577. 1975.


32. Yoon JH, Joo HY, Han JS. A Case of Bilateral Renal Vein Thrombosis in paroxysmal Nocturnal Hemoglobulinuria. Korean Intern Med 30:699. 1986.
34. Kauffmann RH, Veltkamp JJ, Van Tilburg NH, Van ES LA. Acquired Antithrombin III Deficiency and thrombosis in the Nephrotic syndrome. Am J Med 65:607. 1978.


35. Wagoner RD, Stanson AW, Holley KE, Winter CS. Renal vein Thrombosis in Idiopathic Membranous Glomerulopathy and Nephrotic Syndrome: Incidence and Significance. Kid Intern 23:368. 1983.

36. Trew PA, biava CG, Jacobs RP, Hopper J Jr. Renal Vein Thrombosis in Membranous Glomerulonephropathy: Incidence and Association. Medicine 57:69. 1978.


38. Clark RA, Wyatt GM, Colley DP. Renal Vein Thrombosis: An Underdiagnosed Complication of Multiple Renal Abnormalities. Radiology 132:43. 1979.


39. Braun B, Weilemann LS, Weigand W. Ultrasonographic Demonstration of Renal vein Thrombosis. Radiology 138:157. 1981.


41. Bahnson RR, Wendel EF, Vogelzang RL. Renal Vein thrombosis Following Puerperal Ovarian Vein Thrombophlebitis. Am J Obs Gyn 152:290. 1985.

42. Abrans HL. Renal Venography. In: Abrams HL, ed. Angiography. 2nd ed. Little, Brown, Boston: 1971;915.
43. Rowenfield At, Zeman RK, Cronan JJ, Taylor KJ. Ultrasound in Experimental and Clinical Renal Vein Thrombosis. Radiology 137:735. 1980.


44. Miller RA, Tremann JA, Ansell JS. The Conservative Management of Renal Vein Thrombosis. J Urol 111:568. 1974.


46. Reed RR, Carey TC, Hodges CV. Actue Renal Vein Thrombosis with Dextran Treatment. J Urol 118:851. 1977.


47. Andrassy K, Ritz E, bommer J. Hypercoagulability in the Nephrotic syndrome. Klinische Wochenschrift 58:1029. 1980.


48. Burrow CR, Walker WG, Bell WR, Gatewood OB. Streptokinase Salvage of Renal Function after Renal Vein Thrombosis. Ann Intern Med 100:237. 1984.


-
METRICS

- Related articles
-
Loffler`s Syndrome Associated with Clonorchis Sinensis Infestation2003 December;18(4)
Right Atrial Maass Associated with Hepatoma -2 Case Reports-1994 July;9(2)
Empty Sella Syndrome Associated with Central Nervous System Cysticercosis1988 July;3(2)
Hemophagocytic Syndrome Associated with Bilateral Adrenal Gland Tuberculosis2004 March;19(1)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


