INTRODUCTION
A variety of cell types that express Class II histocompatibility (la) antigen on their surfaces are capable of presenting foreign antigens to T lymphocytes1). It is now well documented that macrophages, dendritic cells, vascular endothelial cells, synovial cells, dermal fibroblasts, and B lymphocytes function as antigen presenting cells2ŌĆō5). But articular chondrocytes that are embedded in the complex mesh of the avascular matrix were not thought to have any immune function. Articular cartilage is a protective and an immunologically previleged tissue. Since large molecules are impervious to the cartilage matrix, chondrocytes were considered to be immunologically innert. In fact, it was known, but neglected for a decade, that chondrocytes stimulate allogenic lymphocytes in mixed culture6). Recently, Burmester et al have shown that human chondrocytes from patients with various types of arthritis express class II histocompatibility antigens, and they considered this phenomenon a marker of chondrocytes activation7). This report prompted some investigtors to study the function of la positive chondrocytes as antigen presenting cells8). We undertook this study to confirm whether articular chondrocytes can participate in foreign antigen presentation through an la antigen dependent pathway.
MATERIALS AND METHODS
1. Animals and Immunization
Adult New Zealand White rabbits weighing 1 to 2 kg were obtained from a local breeder. The animals were immunized in each front foot pad with 0.4 mg of OVA in phosphate-buffered salt (PBS) solution emulsified with complete FreundŌĆÖs adjuvant. The animals were sacrificed in 4 week after immunization.
2. Collection of Tissue
Sacrificed rabbits were aseptically dissected. Axillary lymph nodes and spleen were obtained. Both hip and knee joints were opened and femurs with proximal portion of tibia were removed free from adjacent soft tissues. The bones were collected in 50 ml centrifuge tubes (Falcon) containing HBSS.
3. Preparation of Lymph Node Cells and Spleen Cells
Lymph nodes were teased with forceps or needles on petri dishes, and cells were dispersed by repeated pipetting. The cell suspension was spun down and the pellet was treated with distilled water for 15 sec and follwed by additions of 2├ŚNaCI to lyse the erythrocytes. After centrifugation over Ficoll/Hypaque, the cells were washed twice with PBS and adjusted to a cell concentration of 5├Ś106/ml in 5% fetal calf serum in RPMI 1640 containing penicillin, streptomycin and 10 mM HEPES buffer. Lymph node cells were cultured in 100 mm2 petri dishes overnight in a CO2 incubator at 37┬░C to remove adherent cells. Spleen cells were prepared as above and cultured in 25 cm2 flasks. The flasks were placed upright in the incubator.
4. Preparation of Chondrocytes
Dissected bones were washed repeatedly with HBSS. Articular cartilage was shaved with a scalpel from the both ends of femur and the proximal end of the tibia. Care was taken to obtain cartilage without contamination of subchondral bone. Shaved cartilage tissue were placed in 100 mm2 petri dishes and cut into small slivers. The cartilage slivers were transfered to 20 ml of DulbeccoŌĆÖs minimal essential medium (DMEM) containing 2 mg/ml of type II collagenase (Sigma), 0.1 mg/ml hyaluronidase, 0.15 mg/ml DNAse (Worthington), and antibiotics. The dishes were placed overnight at 37┬░C in a CO2 incubator.
The next day the cell suspension was filtered through a Nytex filter (Tetco) with a pore size of 80 ╬╝m, and washed three times with HBSS. Finally chondrocytes were resuspended in serum free DMEM.
5. Purification of T Lymphocytes
Nylon wool purification of T lymphocytes was done as described previously9). Briefly, Lymph node cells or spleen cells in prewarmed HBSS were passed two consecutive nylon wool packed syringe. After 45 min of incubation the non adherent cells were collected and prepared for use.
6. Preparation of Antigen Presenting cell (APC)
Spleen cells and chondrocytes in DMEM in polypropylene tubes were pulsed with 40 ╬╝g/ml of OVA for 90 min at 37┬░C. Following 45 min, the cells were inactivated with 50 ╬╝g/ml of mitomycin-C. After four washes, cells were readjusted in various concentrations in complete medium (10% FBS-RPMI, penicillin, streptomycin, 5 mM glutamine, 10 mM HEPES buffer, and 5├Ś10ŌłÆ5 2-mercaptoethanol).
7. Cell Cultures
A total of 2├Ś105 purified T cells were distrituted to a triplicate set of wells in flat bottomed 96 well plates. Various numbers of antigen pulsed or nonpulsed APCs were added to responding cells in a volume of 0.1 ml per well in complete media. The responding cells were cultured alone or with antigen OVA, or with the mitogen concanavalin A (Con-A). The cells were cultured for 3 days and were pulsed with 0.5 ╬╝Ci of 3H-thymidine for the following 18 hours. The cells were harvested by an automatic cell harveter on to glass filter discs. The discs were dried and transferred to counting vials and 5 ml of scintillation fluid were added. The vials were counted in a liquid scincillation counter (Beckman).
8. Inhibiton of Antigen Presentation by Anti-la Antibody
The APC cultures were incubated for 30 min in the presence of a 25 vol % of monoclonal anti-rabbit la antibody (2C4), a generous gift from Dr. Katherine Knight10), prior to being mixed with responding cells.
RESULTS
1. Immunofluorescence of Enzyme Digested Chondrocytes
The overnight digestion of cartilage readily dispersed and yielded a homogenous population of chondrocytes. The number of chondrocytes obtained from each rabbit ranged between 1 to 4├Ś106. The viability was more than 80% by tryphan blue exclusion. When the chondrocytes were reacted with monoclonal antibody, 2C4, 15ŌĆō40% of rabbit chondrocytes displayed la antigen on their surface. 35 to 60% of the spleen cells stained with 2C4, but fluorescence intensity was quite variable and not uniform.
2. Antigen Presenting Capability of Chondrocytes and Synovial Cells
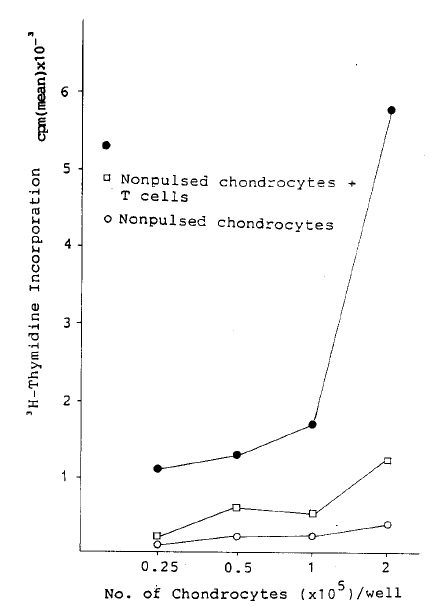
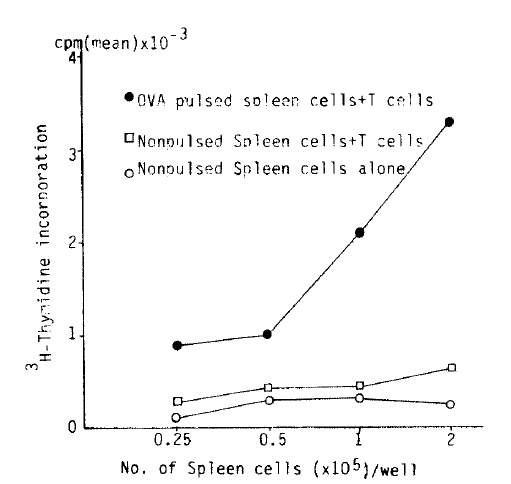
2├Ś105 antigen primed, nylon wool purified T lymphocytes alone did not incorporate a significant amount of 3H-thymidine in the presence of OVA (40 ╬╝g/ml) for 3 day cultures, 0.7┬▒0.2 cpm ├Ś 10ŌłÆ3 (Table 1). However, T lymphocytes in the presence of Con-A incorporated significant amount of 3H-thymidine, 22┬▒3.8 cpm ├Ś 10ŌłÆ3. When T Lymphocytes were incubated with increasing number of antigen non-pulsed chondrocytes, 3H-thymidine incorporation increased from 0.2┬▒0.8 to 1.2┬▒0.3 cpm ├Ś 10ŌłÆ3. Whereas a similar number of OVA pulsed chondrocytes showed an enhancement from 1.1┬▒0.1 to 5.8┬▒0.9 cpm ├Ś 10ŌłÆ3 (Fig. 1). When autologous T lymphocytes were cocultured with an increasing number of antigen nonpulsed and pulsed spleen ceels resulted in incorporation of 3H-thymidine 0.3┬▒0.09 to 0.6┬▒0.2 cpm ├Ś 10ŌłÆ3, 0.9┬▒0.3 to 3.3+1.1 cpm ├Ś 10ŌłÆ3, respectively (Fig. 2). Thus as with the antigen pulsed spleen cells, antigen pulsed chondrocytes function as APC. However, lymph node cells were purified thru two consecutive nylon wool column, the possibility that contaminating antigen presenting cells in responding T lymphocyte population could not be excluded.
3. Inhibition of T Lymphocyte Response by Monoclonal Anti-la Antibody
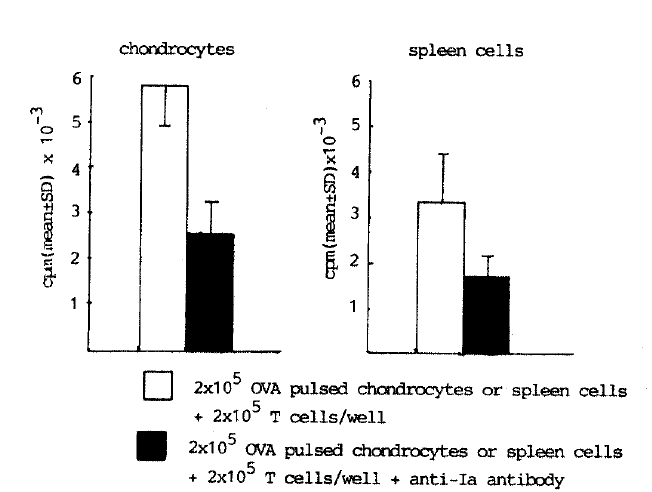
To determine the role of la antigen on chondrocytes in antigen presentation to responding T lymphocytes, chondrocytes were cultured with 25 vol% of mouse anti-rabbit la monoclonal antibody (2C4) after OVA pulsing and mitomycin-C treatment step. With anti la, 55% of inhibition of T lymphocyte response (2.6┬▒0.7 cpm ├Ś 10ŌłÆ3) was observed (Fig. 3). Similar results were obtained from the culture of spleen cells with anti la (Fig. 3). This data suggests that antigen presenting function of chondrocytes is under regulation of la antigen.
DISCUSSION
The results of these experiments demonstrate that articular chondrocytes possess specific antigen presenting ability in common with spleen cells. Moreover, the specific inhibition of autologous T lymphocytes stimulating properties by anti-la antibody adds further evidence that antigen presentation is under restriction of class II antigen.
One marker of the activation of certain cell would be the expression of la antigen. We found 15ŌĆō40% of normal rabbit chondrocytes express la antigen. In contrast, la antigens were found on less than 1% of chondrocytes eluted from normal appearing human cartilage. But the number of la-positive chondrocytes is increased in certain pathologic condition, eg. osteoarthritis, rheumatoid arthritis, and osteochondroma up to 40%7). Furthermore, the cell preparations obtained from these pathologic conditions thru enzyme dispersion, la positive chondrocytes were isolated as free cells without the surrounding matrix. This latter finding suggests that expression of la antigen might indicate a chondrocyte activated and consequently produced matrix degrading enzyme like collagenase to alter its surrounding matrix. Recently, Burmester et al reported that la antigen expression on human articular chondrocytes are inducible by gamma-interferon11). This finding inferred that silent chondrocytes can express la antigen under the direct influence of T lymphocytes. However, the presence of significant percentage of la-positive chondrocytes with normal articular cartilage from rabbits could be due to the difference between species. In our experiment, chondrocytes effectively take up and presented immunized antigen to autologous T lymphocyte population in which accessory cells had been depleted by nylon wool column. But it is possible that the responding lymphocytes used in this study might contain a small number of contaminating macrophages. These macrophages might have provided an accessory cell signal. Another possiblitity that should be considered is the contamination of antigen presenting synovial cells to chondrocyte population during the dissection of cartilage. Recently, Geppert reported that the expression of la antigens and the capacity to take up and degrade antigen effectively are necessary conditions for a cell to function as an APC, but that they are not sufficient. To function as an effective APC, a cell must also have the capacity to engage in interactions, which do not involve the la antigen complex, that react with macrophage or its products like interleukin-112). Furthermore, Minami et al presented the data indicate that for primary class II restricted allo-response, B-lymphocytes produce signals that can be complemented with phorbol ester, suggesting the existance of additional requirements for T lymphocyte activation13). Further dissection of these accessory singals are needed. Extracellular matrix protein, especially type II collagen, have been shown to be immunogenic14), but they have not shown to induce arthritis in rabbit. We may speculated that chondrocytes in normal cartilage are immunologically innert, but under any nonphysiological conditions eg, trauma or infection, they can be exposed, activated and subsequently participate in immune process. Additional studies are now under way to assess la expression on articular chondrocytes at the site of cartilage erosion in situ. However, collapse of lacuna and fibroblastic transformation of chondrocyte at the cartilage erosion site limits the interpretation of immunohistological study.
In summary, articular chondrocytes can take up soluble antigen and result in the stimulation of autologous T lymphocytes. This activity was mediated by class II histocompatibility antigen. Articular chondrocytes can be added to the list of antigen presenting cells like macrophages, endothelial cells, and synovial cells.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





