 |
 |
| Korean J Intern Med > Volume 3(2); 1988 > Article |
|
Abstract
In searching for a new host of the hepadna virus, we found a virus with many of the unique properties of the human hepatitis B virus (HBV) in sera from Manchurian chipmunks inhabiting Korea.
Of 192 tested animals, 8 chipmunks were found to have weak HBsAg reactivities and particles similar in size and shape to the particles assoicated with HBV infection.
The HBsAg-reactive particles contained DNA of similar size to that of HBV and a DNA polymerase activity which appeared to repair a single-stranded region of the DNA molecule.
Histologic examination of liver tissues from chipmunks containing the viral particles in sera showed no evidence of liver disease.
Experimental infection of chipmunk by inoculation of HBsAg-reactive chipmunks sera led to frequent occurence of acute and chronic hepatitis without antigenemia of HBsAg.
Hepatitis B virus (HBV), the prototype human hepadna virus, was identified by the discovery of hepatitis B surface antigen (HBs Ag)1), and its unique characteristics, including its ultrastructure, antigenic makeup, size and structure of DNA, virion DNA polymerase, liver tropism, and features of persistent infection were revealed by extensive subsequent studies.
The virion of HBV (Dane particle) is a double-layered spherical particle approximately 42nm in diameter2) consisting of a lipid-containing outer shell bearing HBsAg and an inner spherical core or nucleocapsid approximately 27nm in diameter. The viral core contains the hepatitis B core antigen (HBcAg)3), the viral DNA4), a DNA polymerase activity5,6), and hepatitis Be antigen (HBeAg) in a cryptic form7).
The viral genome is a circular, partially double-stranded DNA molecule with a length of approximately 3200 base pairs, which has a single stranded region variable in length from 15% to 50% of the circle length in different molecules8,9). DNA polymerase activity in the virion can repair the single stranded region in the viral DNA to make fully double stranded molecule5,6).
Infection with HBV is accompanied by the presence in the blood of Dane particles and much higher concentrations of small spherical particles 22nm in diameter and long filamentous particles with 22nm width and variable length, consisting of excess viral coat protein materials bearing HBsAg2,10).
Persistent infection with HBV is common, continues for many years, and may be associated with chronic hepatitis, cirrhosis, and hepatocellular carcinoma11ŌĆō14).
The host range of HBV is confined to humans, the natural host, and a few other higher primates such as chimpanzees15). The narrow host range and the lack of a tissue culture system for HBV replication has hampered progress in studying viral gene expression and replication.
Recently, three HBV related viruses named wood-chuck hepatitis virus (WHV), ground squirrel hepatitis virus (GSHV), and duck hepatitis B virus (DHBV) have been found in Eastern woodchucks16), Beechey ground squirrels17), and Pekin ducks18). These viruses infecting nonprimates have many features of HBV including similar ultrastructure, partially double stranded DNA of similar size and structure19ŌĆō22), a virion DNA polymerase activity, cross-reacting surface and core antigens23ŌĆō26), common occurence of persistent infection and association with hepatitis and hepatocellular carcinoma27ŌĆō32). HBV and the three related viruses of lower animals are now called the hepadna viridiae33), and such animal viruses have been useful models for the study of replication and pathogenesis of HBV.
In serching for a new host of hepadna virus, we found evidence of a hepadna virus infection in Manchurian chipmunks34).
In this report, we present data showing the evidence and features of this viral infection.
The animals used in this study were apparently healthy Manchurian chipmunks (Tamias sibiricus asiaticus), weighing approximately 80gm, captured from fields in the Kyunggido and Kangwondo provinces of Korea. Sera were obtained from all animals by cardiac puncture and extracted liver tissues were fixed in 10% neutral formalin.
Each serum was tested for HBsAg, anti-HBs, and anti-HBc by radioimmunoassay using commercial kits (Abbott laboratories, North Chicago, III).
To test for the presence of particles similar to HBV in chipmunk sera, sera were pelletted and examined by electron microscope. Each serum sample was centrifuged at 10,000 rpm for 10 min to remove precipitated protein and other debris; 200 ╬╝l of each supernatant diluted with 800 ╬╝l of buffer was layered over an 11 ml gradient of 10ŌĆō20% (Wt/Vol) sucrose containing 10mM Tris-HCl (pH 7.4), 0.1M NaCl, and 5mM EDTA.
After centrifugation for 13 hr at 35,000 rpm in a Spinco SW 40.1 rotor at 20┬░C, the supernatant was thoroughly removed by aspiration, and the pellet was suspended in 100 ╬╝l of the buffer. The suspension was spotted on a carbon-coated grid and the material was stained with 1% phosphotungstic acid and examined by electron microscope.
Pooled sera from Manchurian chipmunks containing HBsAg reactivities and virus-like particles and pooled human sera positive for HBsAg and HBeAg were used for an extensive virion DNA polymerase reaction in the presence of [╬▒-32P] dCTP to radiolabel the viral DNA by the method described by Landers et al9). Serum samples from chipmunks and humans were clarified by centrifugation at 10,000 rpm for 10 min; 4ml of each supernatant was layered over 8 ml of 30% sucrose (wt/vol) in TNEMBSA (10mM Tris-HCl, 0.1M NaCl, 0.001M EDTA, 0.1% 2-mercaptoethanol, and Img of bovine serum albumin per ml, pH7.5) and pelleted in a SW 40.1 rotor for 5 hr at 39,000 rpm and 4┬░C. The pellet was redissolved in 200╬╝l of 0.01M Tris-HCl (pH 7.5), 0.1M NaCl, 0.5% Nonidet P-40, and 0.1% 2-mercaptoethanol.
The 50╬╝l of Tris-phosphate mixture (2.0M Tris-HCl [pH 7.5], 0.08M MgCl2, 0.24M NH4Cl, 1mM each dGTP) dTTP and dATP, 2.5╬╝M [╬▒-32P] dCTP [800 ci/mmol] was added and the reaction mixture was placed at 37┬░C for 20 min. Unlabeled dCTP was then added to a final concentration of 0.2mM and the reaction mixture was incubated for 3hr at 37┬░C.
The reaction mixture was digested with proteinase K in 0.01M Tris-HCl (pH 7.5)-0.01M EDTA-0.15% sodium dodecyl sulfate for 2 hr at 56┬░C. The reaction mixture was extracted three times with phenol equilibrated with TNEME. A one-tenth volume of 2M sodium acetate (pH 5.2) and 2 volumes of 100% ethanol were added to precipitate the DNA. After 2hr at ŌłÆ20┬░C, the ethanol precipitate was pelleted in a Microfuge for 5 min and resuspended in 10mM NaCl-10mM Tris (pH7.5)-1 mM EDTA.
Agarose gel electrophoresis was conducted in a horizontal apparatus using gels of 1% agarose. Gels were run for 12hr at 30V. The size markers were Hind III fragments of bacteriophage lambda DNA. The electrophoresis buffer consisted of 89mM Tris-89mM boric acid-2.5mM EDTA. The gel was subjected to autoradiography at ŌłÆ70┬░C for 120 hr using a Dupont Cronex lightning-plus intensifying screen and Kodak XR-5 film.
Liver tissue fixed in 10% neutral formalin was embeded in paraffin, sectioned at 5 ╬╝m and stained with hematoxylin and eosin.
Each of fifteen Manchurian chipmunks without serologic markers of HBV was inoculated with 100╬╝l of pooled HBsAg-reactive sera from chipmunks. The animals were sacrificed two months later and serologic tests for HBV markers and histologic exams of liver tissues were performed.
Of 192 tested animals, HBsAg reactivities were found in the sera of 8 animals and anti-HBs in 11 animals by radioimmunoassay. Anti-HBc was not detected in any case (Table 1). No HBsAg-positive serum produced more than 10-fold amount of 125I-labeled anti-HBs binding than that of the HBsAg-negative control serum.
All the HBsAg-positive sera contained the particles shown in Fig. 1. The most abundant form in all of the HBsAg-positive sera was a small spherical particle 20ŌĆō22nm (mean 21nm) in diameter. The HBsAg-positive sera also contained doublelayered spherical particles 39ŌĆō42nm (mean 41nm) in diameter with an outer shell and an inner spherical core, similar to the Dane particles of HBV. However, filamentous form of the particle was not observed in any of the HBsAg-positive sera. No such particles were found in HBsAg-negative sera including anti-HBs-positive sera.
The DNA polymerase activity in HBV repairs a single-stranded region in the viral DNA, and the reaction catalyzed by this enzyme can be used to radiolabel the viral DNA and make it fully double stranded. Similarly prepared preparations of particles from HBsAg-positive Manchurian chipmunk and human sera were used for an extensive virion DNA polymerase reaction in the presence of [╬▒-32P] dCTP to radiolabel the DNA and close the single-stranded gap. The DNA was then extracted from each reaction mixture and analyzed by agarose gel electrophoresis and autoradiography.
The results in Fig. 2 show that after an extensive DNA polymerase reaction the radiolabeled DNA from Manchurian chipmunks and human serum particles formed moderately sharp bands with similar electrophoretic mobilities. This form of HBV DNA is fully double-stranded circular molecules9). These results indicate that particles in the HBsAg-positive chipmunk sera contain DNA with a similar size to that of HBV DNA and a virion DNA polymerase that can repair the single-stranded region.
Histologic examination of liver tissues from chipmunks containing HBsAg reactivities and virus-like particles in their sera reavealed no evidence of cell necrosis, infiltration of inflammatory cells or fibrosis (Fig. 3). Chipmunks without HBsAg reactivities showed no evidence of liver diseases, either.
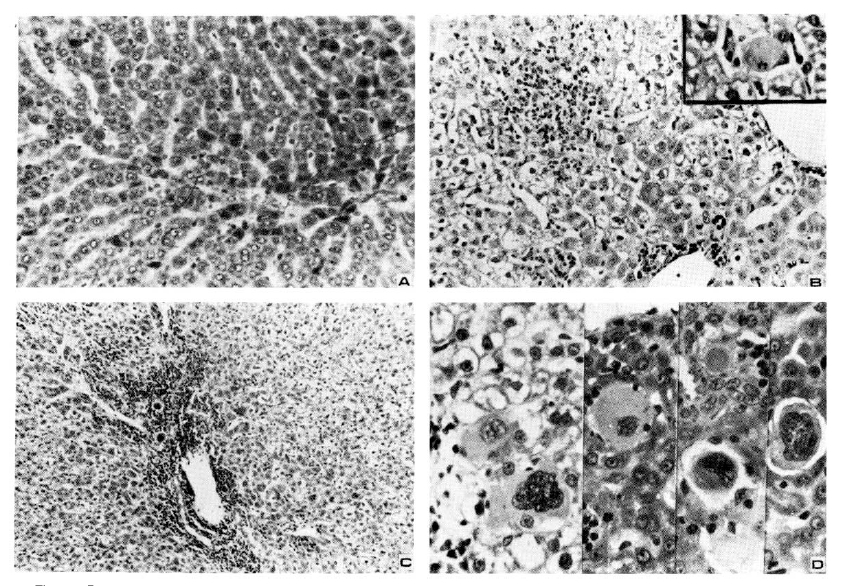
No HBsAg-reactivity was detected in sera obtained from 15 Manchurian chipmunks two months after inoculation with HBsAg-positive sera. However, histologic exams of liver tissues revealed pathologic findings of acute hepatitis in 4 animals, chronic persistent hepatitis in 2, and chronic active hepatitis in 5 including 2 cases showing various appearances of dysplastic hepatocytes with hyperchromatic giant nuclei and ground-glassy ballooned cytoplasms (Fig. 4).
This frequent occurence of significant hepatitis in the inoculated animals is in sharp contrast to the silent histologic findings of the other animals examined immediately after capture without inoculation.
We have found, in the sera of apparently healthy Manchurian chipmunks, particles that have many of the unique characteristics of HBV and related hepadna viuses.
By electron microscopy, double-layered spherical particles 41nm in diameter, similar in appearance to hepatitis B virions (Dane particles), and higher concentration of small spherical particles 21nm in diameter, similar to surface antigen particles of HBV, have been found in all the HBsAg-positive sera from the chipmunks.
Detection of weak HBsAg reactivities in sera of these animals also indicates an antigenic relatedness of the particles to the particles of HBV that have HBsAg reactivity. The low binding of anti-HBs by the particles of the chipmunks may indicate that the chipmunk serum antigen crossreacts with HBsAg but is not identical to it.
The DNA isolated from preparations of HBsAg-positive particles from chipmunks sera appeared to be a circular molecule with similar size to that of HBV and it appeared to contain a single-stranded region which was repaired by a virion DNA polymerase.
The findings described above indicate that some of Manchurian chipmunks inhabiting Korea are infected with a virus that is similar to HBV, WHV and GSHV. Although Manchurian chipmunks belong to the Sciuridae family like woodchucks and ground squirrels, the particles found in the sera of chipmunks appear to be closely related but not identical to virions of WHV and GSHV. The 41nm double-layered particles are smaller than virions of WHV (45nm) and GSHV (47nm) and the filamentous form of particle found in sera associated with WHV and GSHV infection was not observed. Direct comparison of viral antigenic proteins and DNA would be required to determine whether this virus is the same or different from other hepadna viruses.
Infections of all four well-characterized members of hepadna viruses frequently persist for many years and are associated with various levels of liver inflammation and hepatocellular carcinoma. In this study, persistency of infection could not be confirmed since all animals were sacrificed at the initial experiment. Histologic examination of liver tissues from chipmunks containing viral particles in sera revealed no evidence of associated liver disease.
The frequent occurence of acute and chronic hepatitis without antigenemia of HBsAg after experimental infection is quite interesting but inexplicable at present. They may represent liver diseases associated with this viral infection not detected by the assay we have used (Ausria-II, Abbott), which appeared to be insensitive due to weak cross-reaction, or they may be the consequence of immunologic response to allogenic proteins. The presence of dysplastic cells in two cases of chronic active hepatitis is also interesting in relation to the known oncogenic potential of hepadna viruses. Thus, long-term study of chipmunks with natural and experimental infection would be required to clarify the existence of associated liver diseases.
Fig.┬Ā1.
Electron micrograph of particles in HBsAg-reactive sera of chipmunks.
Large arrow; Dane particle-like spherical double shelled particles (41nm)
Small arrow; Small spherical particles (21nm)
Bar represents 100nm
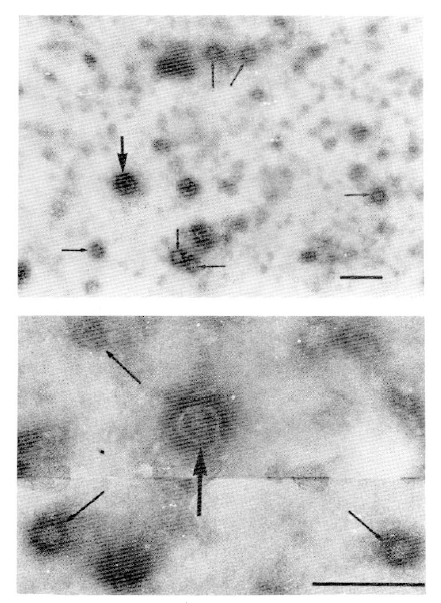
Fig.┬Ā2.
Autoradiogram of [32P] DNA from preparations of HBsAg-reactive particles from Manchurian chipmunk sera (lane A) and from human sera (lane B) after gel electrophoresis. Base pairs are shown on the left.
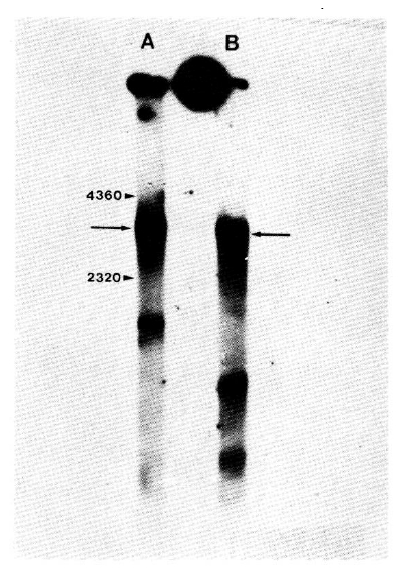
Fig.┬Ā3.
Hematoxylin/Eosin-stained sections of liver from a chipmunk, which contains HBsAg-reactive particles in serum.
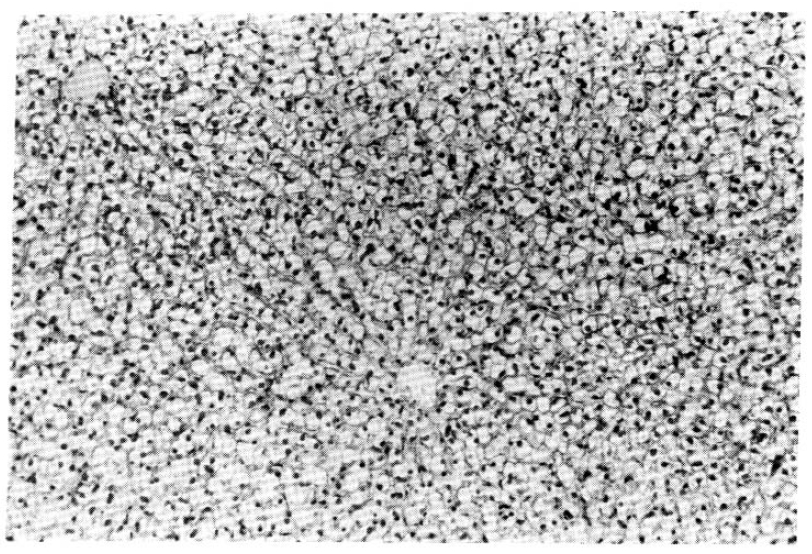
Fig.┬Ā4.
Photomicrographs of liver specimens from chipmunks inoculated with HBsAg-reactive pooled sera.
Acute mild hepatitis showing scattered acidophilic degeneration of hepatocytes
Diffuse, apparently acute, hepatitis showing intralobular accumulations of inflammatory cells, hepatocellular necrosis, ballooning degeneration and acidophilic necrosis (inlet)
Chronic active hepatitis with inflammatory portal space widening, piecemeal necrosis and two dysplastic hepatocytes
Various appearances of dysplastic hepatocytes with bizarre hyperchromatic giant nuclei and ground-glassy ballooned cytoplasms.
REFERENCES
1. Blumberg BS, Alter HJ, Visnich S. A ŌĆ£newŌĆØ antigen in leukemia sera. J Am Med Assoc 191:541. 1965.

2. Dane DS, Cameron CH, Briggs M. Virus-like particles in serum of patients with Australia-antigen-associated hepatitis. Lancet i:695. 1970.

3. Almeida JD, Rubenstein D, Stott EJ. New antigen-antibody system in Australia-antigen positive hepatitis. Lancet ii:1225. 1971.

4. Robinson WS, Clayton DA, Greenman RL. DNA of a human hepatitis B virus candidate. J Virol 14:384. 1974.



5. Kaplan PM, Greenman RL, Gerin JL, Purcell RH, Robinson WS. DNA polymerase associated with human hepatitis B antigen. J Virol 12:995. 1973.



6. Robinson WS, Greenman RL. DNA polymerase in the core of the human hepatitis B virus candidate. J Virol 13:1231. 1974.



7. Takahashi K, Akahane Y, Gotanda T, Mishiro T, Imai M, Miyakawa Y, Mayumi M. Demonstration of hepatitis Be antigen in the core of Dane particles. J Immunol 122:275. 1979.



8. Summers J, OŌĆÖConnell A, Millman I. Genome of hepatitis B virus: Restriction enzyme cleavage and structure of DNA extracted from Dane particles. Proc Natl Acad Sci USA 72:4597. 1975.



9. Landers TA, Greenberg HB, Robinson WS. Structure of hepatitis B Dane particle DNA and nature of the endogenous DNA polymerase reaction. J Virol 23:368. 1977.



10. Bayer ME, Blumberg BS, Werner B. Particles associated with Australia antigen in the sera of patients with leukemia, DownŌĆÖs syndrome and hepatitis. Nature 218:1057. 1968.


12. Szmuness W. Hepatocellular carcinoma and the hepatitis B virus: evidence for a causal association. Prog Med Virol 24:40. 1978.

13. Beasley RP, Lin CC, Hwang LY, Chien CS. Hepatocellular carcinoma and hepatitis B virus: A prospective study of 22,707 men in Taiwan. Lancet ii:1129. 1981.

14. Obata H, Hayashi N, Motoike Y. A prospective study of development of hepatocellular carcinoma from liver cirrhosis with persistent HBV infection. Int J Cancer 25:714. 1980.

15. Barker LF, Chisari FV, McGrath PP, Dalgard DW, Kirschstein RL, Almeida JD, Edgington TS, Sharp DG, Peterson MR. Transmission of type B viral hepatitis to chimpanzees. J Infect Dis 127:648. 1973.


16. Summers J, Smolec JM, Snyder R. A virus similar to human hepatitis B virus associated with hepatitis and hepatoma in woodchucks. Proc Natl Acad Sci USA 75:4533. 1978.



17. Marion PL, Oshiro LS, Regnery DC, Scullard GH, Robinson WS. A virus in Beechey ground squirrels that is related to hepatitis B virus of humans. Proc Natl Acad Sci USA 77:2941. 1980.



18. Mason WS, Seal G, Summers J. Virus of Pekin ducks with structural and biological relatedness to human hepatitis B virus. J Virol 36:829. 1980.



19. Galibert F, Chen TN, Mandart E. Nucleotide sequence of a cloned woodchuck hepatitis virus genome: comparison with the hepatitis B virus sequence. J Med Virol 9:101. 1982.



20. Seeger C, Ganem D, Varmus HE. Nucleotride sequence of an infectious molecularly cloned genome of ground squirrel hepatitis virus. J Virol 51:367. 1984.



21. Mandart E, Kay A, Galibert F. Nucleotide sequence of a cloned duck hepatitis B virus genome: comparison with woodchuck and human hepatitis B virus sequences. J Virol 49:782. 1984.



22. Sprengel R, Kuhn C, Will H, Schaller H. Comparative sequence analysis of duck and human hepatitis B virus genomes. J Med Virol 15:323. 1985.


23. Werner BG, Smolec JM, Snyder R, Summers J. Serologic relationship of woodchuck hepatitis virus to human hepatitis B virus. J Virol 32:314. 1979.



24. Gerlich WH, Feitelson MA, Marion PL, Robinson WS. Structural relationships between the surface antigens of ground squirrel hepatitis virus and human hepatitis B virus. J Virol 36:787. 1980.



25. Feitelson MA, Marion PL, Robinson WS. Antigenic and structural relationships of the surface antigens of hepatitis B virus, ground squirrel hepatitis virus and woodchuck hepatitis virus. J Virol 39:447. 1981.



26. Feitelson MA, Marion PL, Robinson WS. The core particles of HBV and GSHV. I. Relationship between HBcAg and GSHcAg-associated polypeptides by SDS-PAGe and tryptic peptide mapping. J Virol 43:687. 1982.



27. Popper H, Shih JW, Gerin JL, Wong DC, Hoyer BH, London WT, Sly DL, Purcell RH. Woodchuck hepatitis and hepatocellular carcinoma: correlation of histologic with virologic observations. Hepatology 1:91. 1981.


28. Snyder RL, Tyler G, Summers J. Chronic hepatitis and hepatocellular carcinoma associated with woodchuck hepatitis virus. Am J Pathol 107:422. 1982.


29. Popper H, Roth L, Purcell RH, Tennant BC, Gerin JL. Hepatocarcinogenicity of the woodchuck hepatitis virus. Proc Natl Acad Sci USA 84:866. 1987.



30. Marion PL, Knight SS, Salazar FH, Popper H, Robinson WS. Ground squirrel hepatitis virus infection. Hepatology 3:519. 1983.


31. Marion PL, Van Davelaar MJ, Knight SS, Salazar FH, Garcia G, Popper H, Robinson WS. Hepatocellular carcinoma in ground squirrels persistently infected with ground squirrel hepatitis virus. Proc Natl Acad Sci USA 83:4543. 1986.



32. Omata M, Uchiumi K, Ito Y, Yokosuka O, Mori J, Terao K, Wei-fa Y, OŌĆÖConnell AP, London WT, Okuda K. Duck hepatitis B virus and liver diseases. Gastoenterology 85:260. 1983.

33. Robinson WS. Genetic variation among hepatitis B and related viruses. Ann NY Acad Sci 354:371. 1980.


34. Yoo BC, Kim CY. Evidence of a hepadna virus infection in Manchurian chipmunks. Seoul J Med 28:13. 1987.
-
METRICS

-
- 0 Crossref
- 1 Scopus
- 9,082 View
- 48 Download
- Related articles
-
The epidemiology of hepatitis B virus infection in Korea2019 September;34(5)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


