INTRODUCTION
Fulminant hepatic failure (FHF) is defined as encephalopathy due to severe liver dysfunction within 8 weeks of the onset of symptoms, in cases without pre-existing liver disease.1) Histologic features show the liver to have extensive panlobular necrosis of hepatocytes and collapse of the reticulin network.2ŌĆō4)
Its clinical course is serious, resulting in the combined failure of multiple organs. Cerebral edema, renal failure, fluid- electrolytes and acid-base imbalance, respiratory distress, coagulopathy and sepsis could be associated and these could result in death. The reported survival rates are below 20%.5ŌĆō6)
Several animal studies showed prostaglandin E had cytoprotective effects on the mucosal cells of the digestive tract, liver and kidney.7ŌĆō15) Abecassis and his colleagues (1987)16) reported continuous infusion of high does prostaglandin E1 (PGE1) with excellent clinical results in 10 patients with FHF. To our surprise all of these patients lived and recovered completely.
Their result encouraged us to do a therapeutic trial using PGE1 for the treatment of a case of FHF, which had developed after immunosuppressive therapy for aplastic anemia.
CASE REPORT
A 17-year-old high school girl was readmitted to the Department of Internal Medicine, at the National Medical Center on January 14th, 1988 because of vomiting and anorexia. In October, 1987 she had been diagnosed as having severe aplastic anemia, and was treated with intensive immunosuppressive agents (antilymphocyte globulin, prednisone and cyclosporin-A), androgen and multiple blood transfusions (packed RBC and platelet rich plasma).
At that time, her viral markers for the hepatitis B virus (HBV) were all negative. Several weeks later, she had positivity for HBsAg and HBeAg. Multiple blood transfusions may be a possible correlative factor for her having the B virus. She was discharged well and followed regularly via the out-patient department (OPD).
Her peripheral blood count improved progessively (Table 1). One month later, vomiting and anorexia developed, but liver function tests were within the normal range and the symptoms were relieved spontaneously.
However, the symptoms reoccurred and caused her to readmit.
On admission, her mentality was in a state of somnolence and progressed to be drowsy and irritable (stage III encephalopathy), but flapping tremor was absent.
Initial laboratory features are presented in Table 1. The serum transaminase level was elevated to over 3000U/L. HBsAg was negative. Fever, diarrhea, petechiae, ascites, pleural effusion, bleeding tendency and jaundice developed progressively. Abdominal paracentesis revealed that she had hemoperitoneum. All of the supportive care for hepatic encephalopathy, fever and bleeding and prevention of hypoglycemia were provided.
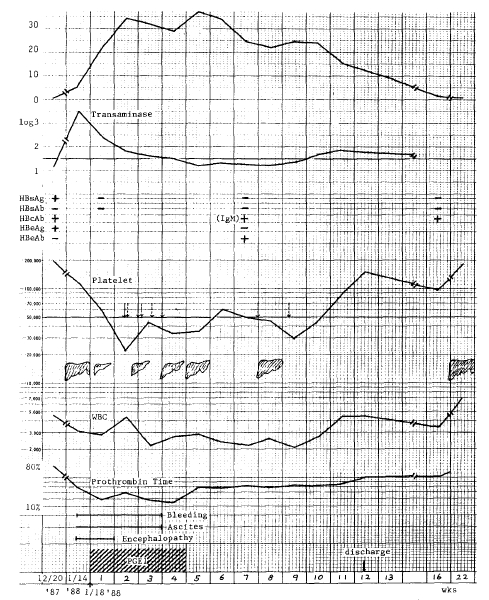
On the 5th hospital day, we started a 24 hour continuous infusion of PGE1 (prostandin®, 20μ/hr) for 28days. Her hospital course and change of laboratory parameters are exhibited in Figure 1.
Her mental status and clinical condition improved after 3 days of PGE1 therapy and were restored to normal on day 7. However, other signs restored to normal on day 7. However, other signs remained for 3 weeks with deepening of the jaundice. Serum total billirubin was increased to 38mg/dl, and fell to the normal level in the 16th week of therapy. Prolonged prothrombin time and hypoalbuminemia persisted, but they returned to normal ranges in the 22nd week of PGE1 therapy.
Pancytopenia reapeared. IgM anti-HBc was positive and perisisted. Alpha-fetoprotein was elevated but rapidly fell close to the normal level.
There were no evidence of DIC and renal insufficiency.
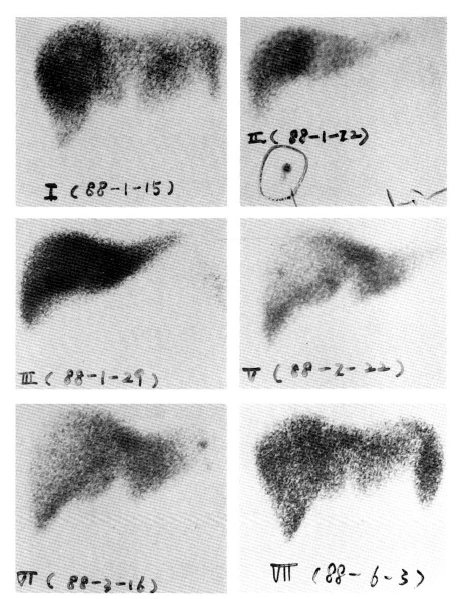
Serial radionuclide liver scintiscans showed progressive recovery of her previously shrunk liver (Fig.1).
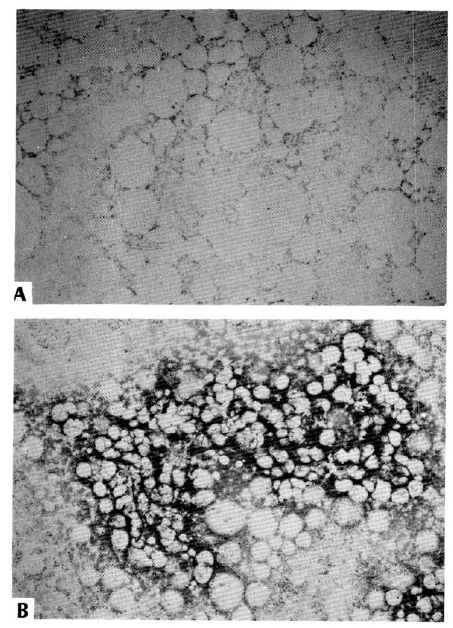
Twelve weeks after PGE1 therapy, she was discharged in good condition, and followed in OPD. Laboratory parameters improved to almost normal ranges (Table 1). Though platelet count recovery was delayed, her bone marrow finding showed normal cellularity with adequate megakaryocytes in number on the 20th week of PGE1 therapy (Fig. 3).
DISCUSSION
The etiology of FHF is reported to be various, including hepatotoxic drugs, idiosyncratic drug reaction, Hepatitis A, B and NANB virus, acute fatty liver during pregnancy, and Budd Chiari syndrome as well as ReyeŌĆÖs syndrome. Hepatitis B virus (HBV) seems to be the most common cause. FHF occurs approximately 1ŌĆō4% in patients with HBV.2ŌĆō4)
Due to the rapid clearance of HBsAg in the case of FHF, diagnosis is dependent on a high titer of IgM anti-HBcAb, which usually persists for I year.17ŌĆō18)
The change of HBV markers observed in FHF suggests that active viral replications stop in an early period. In our case, HBsAg rapidly disappeared, but IgM anti-HBc Ab was positive for 5 months until this report was made.
The early appearance of anti-HBsAb suggests that patients with FHF have a hyperimmune reaction. An excessive immune complex can deposit in the liver and cause hepatic cell necrosis by means of a Type III hypersensitivity reaction.2) Galbraith29) and Thung20) and their colleagues reported a number of cases of FHF occurring after withdrawal of cytotoxic chemotherapy and immunosuppressive therapy in chronic HBsAg carriers with leukemia, choriocarcinoma and Non-HodgkinŌĆÖs lymphoma. In this case, FHF developed in the immune recovery phase after discontinuation of the immunosuppressive agents, such as antilymphocyte globulin, prednisone and cyclosporin A.
Prostaglandins of the E series were known to have cytoprotective effects on mucosal cells of the GI tract. In 1980, Tarawski and his co-workers10) reported a cytoprotective effect of dimethyl PGE2 on damaged liver cells in rats. In other animal studies, the induction of hepatic cell necrosis by murine hepatitis virus strain 3 or CCI4 could be prevented by 16, 16 dimethyl PGE2 or PGE1.9ŌĆō15) Abecassis and his colleagues16) had experience with PGE1 therapy in 10 cases of virus and acetaminophen induced FHF. Their patients received continuous infusion of PGE1, 30ug/hr, for 28 days. They all survived and showed marked improvement in the clinical, biochemical and histologic parameters of FHF.
We treated our patient with a two-thirds dosage of PGE1 (20ug/hr) continuously for 28days, because her body weight was so light (38ŌĆō40Kg).
Infusion of PGE1 was started 5 days after the onset of encephalopathy. Improvement of her general condition and mental status was noted within 3 days of PGE1 infusion, but hemoperitoneum and other symptoms improved later.
Serum transaminase was not thought to be a good parameter for the estimation of liver dysfunction or regeneration because of massive hepatic necrosis brought on by the rapidly decreased enzyme level.
Prothrombin time and serum bilirubin level returned to normal values about 12 weeks later. It was suggested that bile recanaliculization may have been delayed in comparison with the regeneration of hepatocytes. We tried a plasma exchange, hoping to decrease her bilirubin level. There was little effect.
Although a liver biopsy was not done, serial radioisotope imaging of the liver showed progressive regeneration of the liver parenchyma (Fig. 2).
Pancytopenia was observed transiently and may have been due to decreased G-CSF function to GEEM colony forming multipotent stem cells by PGE1.21ŌĆō23)
Although the patient suffered from aplastic anemia and had many poor prognostic features, such as prolonged prothrombin time, high serum bilirubin level and low serum albumin, she recovered and showed improvement of all clinical and biochemical parameters. She also recovered from aplastic anemia with improved bone marrow findings (Fig. 3).
In conclusion, the early intervention of continuous infusion of PGE1 and meticulous supportive care provides hope for cases of acvte FHF. We expect that investigational trials of our modality will be conducted at other institutes.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





