INTRODUCTION
Plasminogen activator is released from endothelial cells by several stimuli, and the amount released may be interpreted as a measure of the fibrinolytic capacity or reserve1) This reserve has been postulated to be reduced in some thrombotic diseases or hypercoagulable states1). One method of assessing endothelial cell plasminogen activator content is a histochemical technique2,3), but this requires biopsy of a vein and is inappropriate for general clinical application. Measurement of the increase in the plasminogen activator content of blood, after stimulation by venous occlusion4) or DDAVP5) is more suitable. Of course, it has been well known that the venous fibrinolytic response is not the same as that of the artery6), and they are both variable depending on the anatomical location of the vessel7). So it is not easy to characterize the fibrinolytic response of one branch of vein in the total fibrinolytic activity of plasma. But it is also well known that the increased portion of fibrinolytic activity with various kinds of stimulation originates from venous endothelial cells8). Thus, we can question the individual variance and distribution of the capacity of fibrinolytic response of vessels as a preliminary study before we start a study of thrombosis related disease. By using stimuli which would give us reproducible response results, this would be a tool to qualify and assess the individual fibrinolytic activity capacity. Using the fibrin plate method9,10), total fibrinolytic activity and the response of fibrinolytic activity to standardized venous occlusion were measured and assessed as to whether they are influenced by age and sex.
MATERIALS AND METHOD
1. Subjects
The participants consisted of 118 healthy volunteers (55 males, 63 females) comprised of 20 medicial students, 7 doctors, 30 nurses and 61 cases who had visited Soonchunhyang Chunan Hospital for a general check-up with the result of no medical problems, especially hypertension, diabetes mellitus, cardiovascular disease and any thrombosis related diseases retrospectively. Their ages ranged from 15 to 75 years (mean 41.2). The range of systolic blood pressure was 110–140 mmHg, and diastolic pressure was 60–90 mmHg. The age distribution of the male group was 16 cases in the 15–30 age group, 15 cases in the 31–45 age group, 14 cases in the 46–60 age group, and 10 cases in the 61–75 age group. The female group consisted of 20 cases in the 15–30 age group, 19 cases in the 31–45 age group, 14 cases in the 46–60 age group, and 10 cases in the 61–75 age group. Any participant who took any medication during the 48 hours before the study, and heavy smokers and heavy drinkers were excluded.
2. Plasma Sampling and Venous Occlusion
The study was done between 8 and 9 AM after overnight fasting. Venous occlusion was produced by application of a sphygmomanometer cuff wrapped round the upper arm and inflated for 5 minutes at 100 mmHg. Blood samples were obtained from an antecubital vein before application of the cuff and again after venous occlusion immediately before the cuff was deflated. The blood samples obtained before the venous occlusion were taken either with or without the help of a tourniquet that had been applied for less than 1 minute. The blood was transferred immediately into plastic tubes containing 2.8% sodium citrate in a 9:1 blood/anticoagulant ratio. Platelet poor plasma was separated by centrifugation at for 30 minutes at 2000×g and stored at −70°C in aliquots of 1.5 ml.
2) Solutions
Plate buffer: The plate buffer, ionic strength 0.15, contained 0.05 mol/L sodium diethyl barbiturate, 0.093 mol/L NaCl, and 1.66 mmol/L MgCl2 and was adjusted to pH 7.8 with HCl.
EDTA buffer: The EDTA buffer, ionic strength 0.15, contained 0.05 mol/L sodium diethyl barbiturate, 0.10 mol/NaCl, 0.25% (wt/vol) gelatin, and 2.7 mmol/L EDTA and was adjusted to pH 7.8 with HCl.
Dextran sulfate solution: Dextran sulfate, 100 mg/L, was dissolved in distilled water.
Sodium flufenamate solution: Flufenamic acid, 0.5 mol/L, was dissolved in an equivalent concentration of sodium hydroxide and was diluted to 14 mmol/L with EDTA buffer.
3) Preparation of Fibrin Plate
The fibrin layer was formed in 100 × 15 mm plastic petri dishes, specially selected for flatness. One aliquot of stock fibrinogen solution (1 %) was thawed and fittered through glass wool, also 2–3 aliquots of thrombin solution were thawed. Filtered 1 % stock solution was diluted to 0.1 % with fibrin plate buffer. Using a Corning repeater syringe, 6 ml of the 0.1 % fibrinogen solution were added to the plate. The plate was swirled to ensure that the solution covered the plate evenly. While moving the plate in a side to side, up and down direction, 200 ul of thrombine solution were added. The plate was covered and left undisturbed for a minimum of 30 minutes.
3) Preparation of Euglobulin
The test samples and controls were thawed at room temp, and placed on ice as soon as they had thawed. A dextran sulfate euglobulin fraction was prepared in a 16 × 100 disposable glass tube as follows:
Plasma (0.5ml) was diluted with 4.0 ml cold distilled water and 0.5 ml dextran sulfate solution was added. It was mixed and placed on ice and the pH was adjusted to 5.9 (5.85–5.9) with acetic acid solution with a constant stirring motion. It was left to stand on ice for 30–60 minutes, then centrifuged at 2000g for 10 minutes in sorvall at 4°C. The supernatant was decanted completely and the interior of the tube was dried with absorbent tissue. The precipitate was dissolved in 0.5 ml saline barbital buffer.
4) Assay of Plasma Total Fibrinolytic Activity
On a single fibrin plate, three 30 μl drops of dextran sulfate euglobulin fraction were placed onto the surface of the fibrin layer in a triangular fashion. To each drop, 5 μl of sodium flufenamate solution was added directly. The plate was left undisturbed for several minutes to allow absorption of the drop into the fibrin layer and was then incubated at 37°C for 17 hours. Two perpendicular diameters of each lysed zone were measured in mm2. Using a TFA program, the final result was computed in activator units.
5) Statistical Analysis
Before the study started, it was determined that each age group had at least 10 cases in both sexes. Individual data of fibrinolytic activity were converted to blood activator units (BAU) by standard pooled plasma each time. The mean of each group was expressed as mean ± 1 SD. To compare the results between various age groups in both sexes a one-way analysis of variance was performed on the data of basal, stimulated and incremental fibrinolytic activity. The data were compared between the male and female groups with the unpaired t-test. The correlation between age and basal activity or incremental activity after stimulation was analyzed by linear regression.
RESULTS
1. Reproducibility of Basal Fibrinolytic Activity and the Response of Fibrinolytic Activity with Venous Occlusion
Fig. 1 shows the results of fibrinolytic response to venous occlusion for 5 minutes at 100 mmHg from the left anterior cubital vein. Five consecutive studies were performed at a 2 or 3 day interval on a 41-year-old male volunteer. The mean of basal and stimulated fibrinolytic activity were 101.6 ± 0.9 and 121.6 ± 1.9 BAU, respectively and the increment of fibrinolytic activity with standardized venous occlusion was 20 ± 2.6 BAU.
2. Dose-Response Curve
An average dose-response curve was constructed according to the principles described by Kluft.10) Individual assays of serially diluted dextran sulfate euglobulin fractions from 20 healthy volunteers (aged 21 to 60) and an additional 70 dextran sulfate euglobulin fractions from normal pooled plasma, made of 10 healthy male volunteers (aged 31 to 41 years), resulted in linear dilution curves with an average slope of 0.43 (95% confidence limit 0.41 and 0.45). The mean of the 100% concentration of these normal plasma pools was arbitrarily set at 100 BAU/ml (corresponding to a mean of mm2 and a 95% confidence limit of 349 and 405 mm2). Such plasma pools were found to be stable for several months at −70°C. The resulting linear dilution curve with defined slope and position was then used as a dose-response curve for interpolation of individual total fibrinolytic activity.
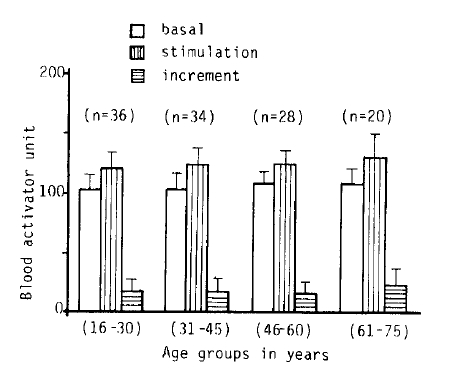
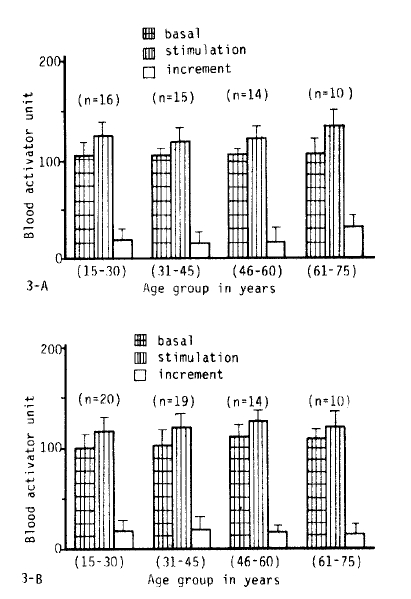
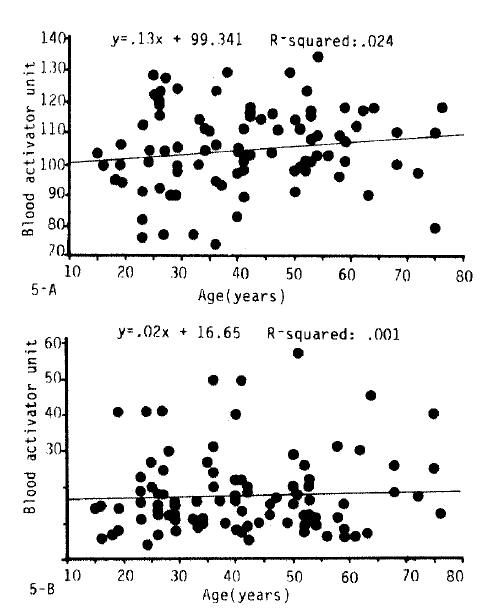
3. The Influence of Age on Fibrinolytic Activity
Fig. 2, 3-A.B, 5 and 6 show the fibrinolytic response on standardized venous occlusion of various age groups. The data show that the influence of age on fibrinolysis was negligible both in the male and female groups. The basal fibrinolytic activities were 102.7 ± 13.2 BAU in the 15–30 age group, 103.3 ± BAU in the 31–45 age group, 108.2 ± 10 BAU in the 46–60 age group, and 107.7 ± 13 BAU in the 61–75 age group. The increments after stimulation were 17.5 ± 10.2 in the 15–30 age group, 17.2 ± 11.6 BAU in the 31–45 age group, 16.2 ± 10.4 BAU in the 46–60 age group and 23.9 ± 14.7 BAU in 61–75 age group. The analysis of variance of these data shows no significant difference between the various age groups (p>0.25).
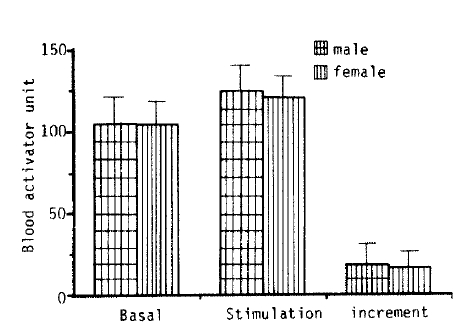
4. The Influence of Sex on Fibrinolytic Activity
Fig. 4 shows that there was no difference in basal fibrinolytic activity and the response of stimuli between the male and female groups (0.1<p<0.375).
DISCUSSION
Our results indicate that there was no difference in the fibrinolytic response to venous occlusionn of the arm with age and sex.
Endothelial cells synthesize and secrete physiologically important substances including those relating to procoagulants11), antiplatelets12), anticoagulants13), and fibrinolysis14). Concerning the fibrinolytic properties, endothelial cells synthesize and secrete not only plasminogen activator but also the inhibitor of it15).
Euglobulin fibrinolysis study is based on the fact that it contains considerably less inhibitors of the fibrinolytic system than does plasma, allowing the detection of fibrinolytic activity normally suppressed by the inhibitors in plasma. But still, euglobulin contains considerable amounts of C1 inactivator and inter-A-trypsin inhibitor9,10). In order to minimize this unwanted inhibitor, we followed Kluft’s suggestion10) of using flufenamate to eliminate C1 inactivator in euglobulin fractions. A limitation of fibrinolysis studies using euglobulin fractions has been that they measure only the spontaneous fibrinolytic activity of blood, which is generally low and unsuitable for demonstrating individual variation of fibrinolytic activity. Fibrinolytic response to venous occlusion of the limbs has been regarded as a sensitive and reliable method for assaying the individual fibrinolytic capacity16–20). But there is controversy as to whether or not the fibrinolytic capacity to venous occlusion varies with age in healthy volunteers; Robertson16) found that fibrinolytic response increased with age, but Isacson and Nilsson17) found no significant variation in the fibrinolytic release capacity with different ages and sex. There may be some reasons for this controversy-many investigators used the fibrinolytic area itself as the fibrinolytic capacity, with which it is not easy to decrease the interassay variance, and the sizes and age distributions of the study groups were not sufficient to analyse the results statistically. Also, previous studies16–20) have occluded the limbs for 15–20 minutes at a level midway between the systolic and diastolic blood pressure, but we standardized the occlusion for 5 minutes at constant pressure (100 mmHg) for assaying the individual fibrinolytic response capacity. This was done for the following reasons: 1) most people can not tolerate such a long occlusion at 100mmHg because of pain, 2) 5 minute occlusion gave us a reproducible fibrinolytic response in our preliminary study (Figure 1), 3) we were afraid of intrinsic or systemic activation of fibrinolytic activity through an unknown pathway from such a long painful compression of the extremity, and 4) we occluded the arm at constant pressure in the hope of homogening the strength of stimulation. Of course, there might be the possibility of inappropriate stimulation to remove the variance of the individual fibrinolytic activity capacity by this standardized occlusion. But we have found that the result was meaningful in specific conditions, for example, it was low in long-standing diabetes mellitus, hypertension, snake bite and hemorrhgic fever (not published yet). In conclusion our results indicate that there was no difference in the fibrinolytic response to venous occlusion of the arm with age and sex.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





