 |
 |
| Korean J Intern Med > Volume 4(1); 1989 > Article |
|
Abstract
Bronchial hyperreactivity is a characteristic feature of bronchial asthma. Recent respiratory infections, allergic rhinitis, atopic family history, pulmonary tuberculosis, pulmonary sarcoidosis, cystic fibrosis, and farmer’s lung have also been demonstrated to have bronchial hyperreactivity to inhaled methacholine. It is not known if pulmonary tuberculosis can cause nonspecific bronchial hyperreactivity and what the mechanism would be. We therefore undertook to evaluate nonspecific bronchial hyperreactivity in active pulmonary tuberculosis using the bronchial provocation test with methacholine and we measured the total serum IgE and peripheral eosinophil count to seek some mechanisms.
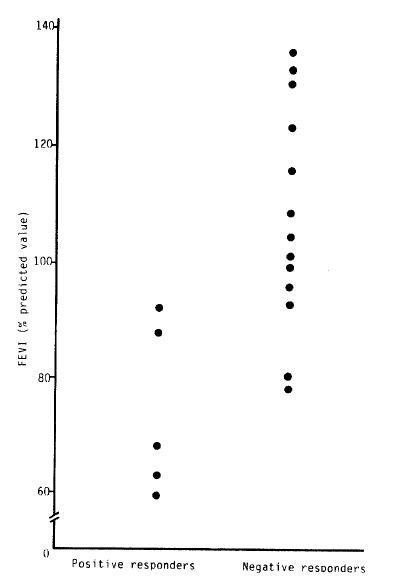
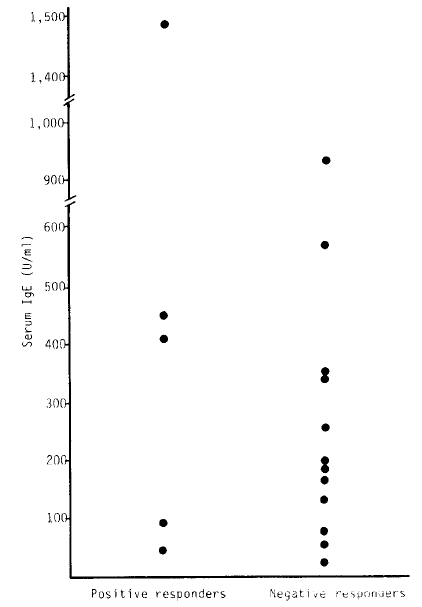
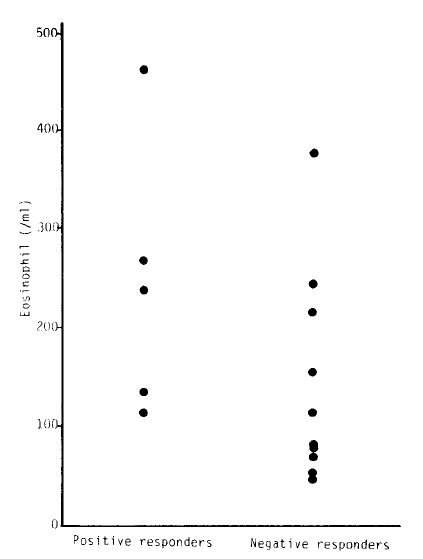
There were 5 patients among 18 subjects with active pulmonary tuberculosis whose response to methacholine was positive. The mean baeline FEV1 of positive responders was 71.40 ± 17.39%, and that of negative responders was 110.18 ± 17.65%(p<0.05). There were no significant differences in serum IgE and peripheral eosinophil count between positive and negative responders.
We found that active pulmonary tuberculosis would increase the nonspecific bronchial response with methacholine, and the mechanism of the bronchial hyperreactivity in patients with active pulmonary tuberculosis may not be related to an immunologic mechanism but may be related to the stimulating receptors.
Bronchial hyperreactivity is a characteristic feature of bronchial asthma and there are allergen-specific and nonspecific bronchial hyperreactivities. Allergen-specific bronchial hyperreactivity is only observed in extrinsic asthma, but nonspecific bronchial hyperreactivity is increased by several factors, including exposure to offending allergens. This is often found in chronic obstructive lung disease and can be caused transiently by viral upper respiratory infections and vaccination of influenza virus6–8). There were some reports that bronchial hyperreactivity can be a manifestation of bronchiectasis8), pulmonary sarcoidosis10), cystic fibrosis11) and pulmonary tuberculosis9).
Several mechanisms have been reported to explain nonspecific bronchial hyperreactivity, but none of them has been clearly identified. It is not determined whether pulmonary tuberculosis can cause nonspecific bronchial hyperreactivity and what the mechanism is. Bronchial provocation tests with methacholine, histamine, or the physical stimuli of exercise are useful in the assessment of nonspecific bronchial hyperreactivity5).
The aim of the present study was to examine nonspecific bronchial hyperreactivity in active pulmonary tuberculosis, using bronchial provocation tests with methacholine, which is a parasympathomietic drug that produces bronchial smooth muscle contraction, and we measured total serum IgE and peripheral eosinophil count to evaluate some mechanisms.
Eighteen patients with active pulmonary tuberculosis participated in this study. This group consisted of 12 men and 6 women, 18 to 54 years of age (mean age 27.5 years) with basal FEV1 over 60% predicted (pred) value and FVC over 80% pred value.
The selected subjects were proven to have active tuberculosis with sputum AFB, bronchoscopic washing AFB, or bronchoscopic biopsy. The patients with a history of previous respiratory allergy or pulmonary tuberculosis medication were excluded.
The potential subjects underwent a physical examination and pulmonary function test, including complete spirometry, before and after inhalation of methacholine.
The severity of the pulmonary tuberculosis lesion was determined by chest X-ray and we checked the serum IgE level and serum total eosinophil count with the ELISA technique.
In our laboratory, a 20% or greater reduction in FEV1 constituted a positive response to methacholine.
After inhalation of normal saline as a basal study, serial administration of the methacholine aerosol with a nebulizer was begun with an FEV1 maneuver performed after each dose (0.31, 0.62, 1.25, 2.5, 5.0, 10.0, and 25.0 mg/ml). Three and a half minutes following inhalation of five deep breaths of the diluent, the pulmonary function studies were repeated.
The test was terminated when the FEV1 had decreased 20% or more from the patient’s baseline or in the absence of a positive response when the dosage schedule was complete.
If the test was negative, five breaths of the next dilution was given at 5 minute intervals. Finally, a bronchodilator aerosol (e.g. salbutamol inhalator) was administered to reverse the bronchoconstrictive effects of methacholine if the test appeared positive.
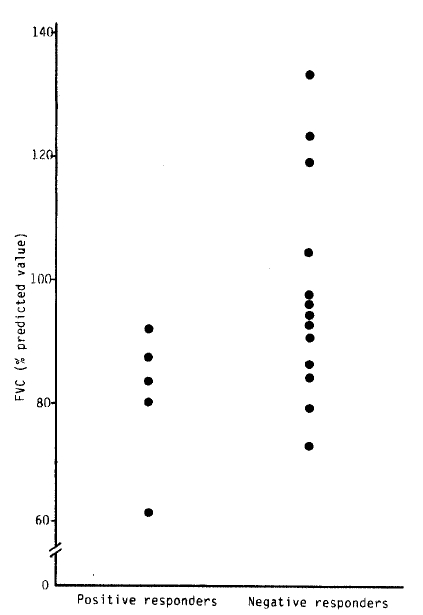
According to the lesions of pulmonary tuberculosis, 3 groups were defined: subjects with minimal pulmonary tuberculosis (n = 6) showed a mean FEV1 of 118% pred (64–140% pred) and mean FVC of 103.7% pred (86–120% pred); subjects with moderate pulmonary tuberculosis (n = 7) showed a mean FEV1 of 97.6% pred (64–140% pred), and a mean FVC of 92% pred (65–126% pred); and subjects with far advanced pulmonary tuberculosis (n = 5) had a mean FEV1 of 79.2% pred (64.117% pred) and a mean FVC of 82.2% pred (49–91% pred).
Five patients showed positive responses to the methacholine bronchial provocation test among 18 patients with active pulmonary tuberculosis. All patients with minimal pulmonary tuberculosis showed negative responses to the bronchial provocation test with methacholine.
But 2 patients among 7 subjects with moderate pulmonary tuberculosis and 3 patients among 5 subjects with far advanced pulmonary tuberculosis revealed positive responses to the methacholine bronchial provocation test (Table 2).
Mean total IgE values were 529.90 U/ml (SD = 524.26) in positive responders and 297.25 U/ml (SD = 264.16) in the negative ones.
The bronchial inhalation challenges with pharmacologic and antigenic substances were introduced to North American for the detection of airway hypersensitivity and hyperreactivity in the 1940’s1–4). The successful application of inhalation challenge techniques has improved our understanding of pathophysiologic and immunologic mechanisms of reactive airway disease5).
Although most subjects with current asthma are exquisitively sensitive to methacholine, bronchial reactivity can be demonstrated in the individuals without asthma.
Recent respiratory infections6), allergic rhinitis2), an atopic family history7), chronic pulmonary disease8), pulmonary tuberculosis9), pulmonary sarcoidosis’10), cystic fibrosis, and farmer’s lung12) have all been demonstrated to influence the bronchial response to inhaled methacholine.
When patients with hyperreactive and hypersensitive airways are exposed to aerosolized pharmacologic bronchoconstriction substances or antigens to which they have become senitized, they will usually develop one of 3 responses, i.e. immediate response, late reponse, and combined response’13).
Possible causes for the exaggerated smooth muscle contraction in bronchial hyperreactivity include a decrease in baseline airway caliber, an increased responsiveness of the smooth muscle itslef, an abnormality in the autonomic stimulus to the target cells”14). The age also has a significant effect on the methacholine responsiveness, that is, younger and older subjects may exhibit a bronchial response that may falsely suggest hyperreactive airway disease18). But the age group of 20 to 60 years does not demonstrate a significant age effect18,19). The age group in this study was 18 to 54 years, so there was no significant age affect.
Three pharmacologic agents are currently used for bronchial inhalation challenges and they are methacholine, carbachol, and histamine. Methacholine is a parasympathomimetic drug that produces bronchial smooth muscle contraction when delivered in sufficient dose by aerosol. Methacholine is slowly broken down in the body by cholinesterase, while carbachol is not significantly inactivated by the enzyme. Inhaled parasympathomimetic drugs produce their effect on the airways by stimulating acetylcholine receptors in bronchial smooth muscle cells15–18,21,22).
The most common parameter of spirometry appears to be the one second forced expired volume (FEV1) for clinical bronchial reactivity testing. In this study we used the FEV1 as a parameter for bronchial hyperreactivity evaluation.
In summary, the resonse to methacholine was positive in five patients among 18 subjects with active pulmonary tuberculosis (2 patients among 7 subjects with moderate pulmonary tuberculosis and 3 patients among 5 subjects with far advanced pulmonary tuberculosis). The mean baseline FEV1 of positive responders was significantly lower than that of negative responders to the bronchial provocation test. There were no significant differences in serum IgE and eosinophil count between positive and negative responders.
In this study, we found that active pulmonary tuberculosis would increase the non-specific bronchial response with methacholine, and the responses were prominent in the subjects with lower FEV1. Because there were no significant differences in the serum IgE and blood eosinophil count between the 2 groups, we suggest that the mechanism of bronchial hyperreactivity in subjects with active pulmonary tuberculosis may not be an immunologic mechanism but may be related to the stimulating receptors.
However, a bronchial provocation test with extracts of tubercle bacilli or long term follow-up studies for the attended subjects are necessary to elicit the more exact mechanism of bronchial hyperreactivity in patients with activie pulmonary tuberculosis.
Table 1.
Characteristics of the Subjects
Table 2.
Results of the Bronchial Provocation Test.
| Bronchial provocation Severity of the lesion | Positive | Negative |
|---|---|---|
| Minimal | 0 | 6 |
| Moderate | 2 | 5 |
| Far advanced | 3 | 2 |
|
|
||
| Total | 5 | 13 |
Table 3.
Comparison of Bronchial Provocation Prositive and Negative Groups for each Parameter
| IgE (ELISA) (U/ml) | Eosinophil (U/ml) | FEV1 (% pred) | FVC (% pred) | |
|---|---|---|---|---|
| Positive | 529.90 ± 524.26 | 260.60 ± 104.72 | 71.40 ± 17.39 | 77.8 ± 14.99 |
| Negative | 297.25 ± 264.16 | 164.64 ± 125.36 | 110.18 ± 17.65 | 99.08 ± 17.42 |
| t-value | 0.9835 | 1.4847 | 3.7579** | 2.4005** |
REFERENCES
1. Curry JJ. The action of histamine on the respiratory tract in normal age and asthmatic subjects. J Clin Invest 25:785. 1945.



2. Curry JJ. Comparative acetyl-beta-methacholine and histamine on the respiratory tact in normals, patients with hay fever and subjects with bronchial asthma. J Clin Invest 26:430. 1947.



3. Schiller IW, Lowell FC. The effects of drugs in modifying the response of asthmatic subjects to inhalation of pollen extracts as determined by the vital capacity measurements. Am Allergy 45:564. 1947.
4. Lowell FC, Schiller IW. Measurement of changes in vital capacity as a means of detecting pulmonary reactions to inhaled aerosol allergic extracts in asthmatic subjects. J Allergy 19:100. 1948.


5. Guidelines for bronchial inhalation challenges with pharmacologic and antigenic agents. ATS News Spring 11. 1980.
6. Empey DW, Laitinen LA, Jacobs L, Gold WM, Nadel JA. Mechanisms of bronchial reactivity in normal subjects after upper respiratory tract infection. Am Rev Respir Dis 113:131. 1976.

7. Townley RG, Bewtra AK. Airway reactivity to methacholine in asthma family members and twins. In: Spector S, ed. Provocative Challenge Procedures: Bronchial, Oral, and Exercise. 177. Boca Raton Fla: CRC Press Inc, 1983.
8. Bahous J, Cartier A, Quimett G, Pineau L, Malo JL. Non-allergic bronchial hyperexcitability in chronic bronchitis. Am Rev Respir Dis 129:216. 1984.

9. Latitinen LAL. Histamine and methacholine challenge in the testing of bronchial reactivity. Scand J Respir Dis Suppl 86. 1974.
10. Laitinen LA, Haahtela T, Kava T, Laitinen A. Nonspecific bronchial reactivity and ultrastructure of the airway epithelium in patients with sarcoidosis and allergic alveolitis. Eur J Respir Dis 64(Suppl):131. 264–2841983.

11. Varpela E, Laitinen LA, Keskinen H, Korhola O. Asthma, allergy and bronchial hyperreactivity in patients with bronchiectasis. Clin Allergy 8:273–2801978.


12. Monkare S. Cinical aspects of farmer’s lung: airway reactivity, treatment and prognosis. Eur J Respir Dis 65(Suppl):65. 1984.
13. Yan K, Salome C, Woolcock J. Rapid method for measurement of bronchial responsiveness. Thorax 38:760. 1983.



14. Boushey HA, Holtzman MJ, Nadel JA. Bronchial hyperreactivity. Am Rev of Resp dis 121. 1980.
15. Rosenthal RR, Normal PS, Summer WR, Permutt S. Role of the parasympathetic system in antigen-induced bronchospasm. J Appl Physiol Resp Environ Exercise.

16. Fish JE, Rosenthal RR, Summer WR, Menkes H, Normal PS, Permutt S. The effect of atropine on actue antigen mediated airway constriction in subjects with allergic asthma. Am Rev Respir Dis 115:371. 1977.

17. Townley RG, McGready S, Bewtra A. The effect of beta adrenergic blockade on bronchial sensitivity to acetyl-beta-methacholine in normal and allergic rhinitis subjects. J Allergy 57:358. 1976.

18. Hopp RJ, Bewtra A, Nair NM. The effect of age on methacholine response. J allergy Clin Immunol October 609. 1985.

19. Boushey HA, Holtzman MJ, Sheeler NR, Nadel JA. Bronchial hyperreactivity. Am Rev Resp Dis 121:389–4131980.

20. Cockcroft DW, Bersheid BA. Measurement of responsiveness to inhaled histamine: Comparison of FEV1 & SGaw. Annals of Allergy 51:Spe. 374. 1983.
-
METRICS

- Related articles
-
Survival and Prognostic Factors in Patients with Primary Pulmonary Hypertension2001 June;16(2)




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


