 |
 |
| Korean J Intern Med > Volume 7(1); 1992 > Article |
|
Abstract
Background
Prostaglandin E which is present abundantly in the gastric mucosa is a powerful inhibitor of gastric acid secretion and a stimulus to gastric mucus production. In addition, prostaglandin E2 inhibits ulcer formation in animals, and the synthetic analogues of prostaglandin E have successfully been used in the treatment of patients with gastric and duodenal ulcer disease. To evaluate the role of endogenous prostaglandin E2 in the pathogenesis of the peptic ulcer disease, we measured mucosal prostaglandin E2 levels in patients with gastric and duodenal ulcer disease and compared with that of non-ulcer control persons.
Methods
The study population was made up of 44 non-ulcer persons, 36 patients with a benign gastric ulcer, and 48 with a duodenal ulcer. Every mucosai specimen, taken from the antrum and from the duodenal bulb, were homogenized, mixed with 1 M HCI, and centrifuged. After removal of the supernatant, precipitate was eluted with ethyl acetate in the Amprep C18 minicolumn. Then the extracted prostaglandin E2 in the ethyl acetate fractions was converted into its methyl oximate derivatives, and the prostaglandin E2 level was measured by radioimmunoassay. During the procedure any homogenized specimen which was looking grossly bloody was removed from the assay in order to avoid any possible contamination or prostaglandin E2 in blood.
Results
In non-ulcer persons, the mean values was 258.17┬▒127.03 pg/mg. tissue in antrum and 121.07┬▒67.46 pg/mg. tissue in duodenal bulb. The corresponding values were 186.42┬▒70.51 pg/mg. tissue, 79.44┬▒39.04 pg/mg. tissue in gastric ulcer patients and 204. 94 92.03 pg/mg. tissue, 99.66┬▒56.10 pg/mgl. tissue in duodenal ulcer patients respectively. Gastric ulcer patients have the significantly lower level of the antral and duodenal prostaglandin E2 (p<0.005). Those levels of duodenal ulcer patients were also significantly lower than those of non-ulcer persons (p<0.025 & 0.05). Antral prostaglandin E2 level increased to 305.21┬▒104.91 pg/mg. tissue in the gastric ulcer patients (p<0.005) and to 271.02┬▒93.23 pg/mg. tissue in the duodenal ulcer (p<0.005) when the ulcer crater was healed. The duodenal bulb prostaglandin E2 level was also increased in the healed stage of ulcer, e. g., 128.84┬▒57.62 (p<0.005) and 112.60┬▒42.25 pg/mg. tissue, respectively.
Prostaglandin E2 (PGE2) which is produced and stored in the gastric and duodenal mucosa1ŌĆō3) is a powerful inhibitor of gastric acid and pepsin secretion4,5) and a stimulus of gastric mucus6) and gastroduodenal bicarbonate secretion7,8). In addition, PGE2 has been shown to inhibit ulcer formation in animals9,10), and the orally effective PGE2 analogues have successfully been used in the treatment of patients with gastric and duodenal ulcer disease11ŌĆō13). Moreover, inhibition of endogenous prostaglandin synthesis causes gastrointestinal ulcers14,15), while stimulation of prostaglandin synthesis protects against mucosal injury16,17). From these experiments it might be expected that gastric and duodenal ulcer disease would be associated with reduced tissue levels of prostaglandins18ŌĆō20). The present study was aimed to evaluate the possible role of endogenous PGE2 in the pathogenesis of peptic ulcer disease by comparing the tissue levels of PGE2 in an active ulcer and after healing of the ulcer disease.
The study population was made up of 44 healthy persons with no previous history of peptic ulcer or G-l bleeding disorders. Of them, 23 patients were male and 21 were female. Their average age was 43.8 years for males(range 24ŌĆō67 years) and 36.7 for females (range 22ŌĆō52 years). Active ulcer craters and their benignancy were confirmed by both endoscopy and histopathologic examination of each biopsy specimen. Gastric ulcer patients consisted of 27 male (range 32ŌĆō72 years, average 51.2 years) and 10 female patients (range 32ŌĆō68 years, average 45.5 years). Thirty six male patients, average age 42.0 years (range 18ŌĆō68 years) and 12 female patients, average age 43.3 years (range 24ŌĆō58 years) were included in the duodenal ulcer group (Table 1).
Gastro-duodenal endoscopy was performed at least-after a 12 hour fast with the Fujinon EVG-FP (Japan) endoscope. On the basis of the endoscopic findings the patients were divided into (1) non-ulcer; (2) active gastric ulcer; (3) active duodenal ulcer; and (4) healed ulcer (definite scarring of the previously visualized ulcer crater). Four biopsy specimens were obtained from the gastric antrum and the duodenal bulb before the beginning of the anti-ulcer treatment and after the healing of the ulcer, respectively. Antral mucosa were taken from the lesser curvature site of the antrum approximately 5 cm from the pyloric ring and duodenal mucosa were obtained from the normally appearing site of the duodenal bulb.
All biopsy specimens taken from each anatomical site were measured, weighed and transferred without delay into 0.2 ml of phosphate buffered saline(PBS 0.1 M, pH 7.4), homogenized, and kept under the ŌłÆ70 C until the PGE2 assay. Any homogenized specimen which was looking grossly bloody was removed from the assay in order to avoid any possible contamination of PGE2 in blood. All PGE2 assay were performed within 7 days after preparation.
Into a microfuge 1.5 ml polypropylene tube, 0.2 ml of homogenate of mucosal specimen and 0.6 ml of 1 M HCI were mixed gently and left at room temperature for 5 minutes. Each sample was centrifuged at 2,500 g for 5 minutes, and the supernatant was removed. Then the precipitate was applied to an Amprep C18 minicolumn which has been primed with 2 column volumes of 10% ethanol. The column was washed with 1 column volume of distilled water and 1 column volume of hexane. The PGE2 was eluted with 2├Ś0.75 ml ethyl acetate and the ethyl acetate fractions were evaporated to dryness under nitrogen. Prior to analyzing extracted samples, following procedures were performed. One hundred micro liter of phosphate buffered gelatin saline (pH 7.0) and 100 micro liter of methyl oxymation reagent were added to the dried fractions, mixed gently and incubated at room temperature for overnight. Then the concentration of PGE2 was determined by Prostaglandin E2 [125I] Assay System. (Amersham International plc, Amersham, UK)
In the 44 non-ulcer persons studied, the anrum contained 258.17┬▒127.03 (97.14ŌĆō548.83) pg/mg. tissue and the duodenal bulb 121.07┬▒67.46 (45.00ŌĆō357.14). The antrum contained significantly higher concentration of PGE2 than the duodenal bulb (p<0.005) (Table 2 and Fig. 1). In the 37 patients with benign gastric ulcer the antrum contained 186.42┬▒70.51 (55.71ŌĆō396.55) pg/mg. tissue and the duodenal bulb 79.44┬▒39.04 (32.64ŌĆō202.50). These were also significantly different from each other (p<0.005). In gastric ulcer patients PGE2 concentrations of both the antral and the duodenal bulb were significantly lower than the respective levels in the non-ulcer persons (p<0.005) (Table 2 and Fig. 1). In the 48 patients with active duodenal ulcer the PGE2 concentration were 204.94┬▒92.03 (39.68ŌĆō400.00) pg/mg. tissue in the antrum and 99.66┬▒56.10 (36.36ŌĆō291.43). in the duodenal bulb. These were also significantly different from those of non-ulcer persons (p<0.025 & p<0.05) (Table 2 and Fig. 1). All patiets with either the gastric or the duodenal ulcer were completely healed after 4 to 7-week treatment with the H2-receptor blocking drug (Ranitidine 300 mg/day, at bed time) in combining with or without the liquid antacid (Mylanta 40 ml to 70 ml a day). There was no significant difference between the location of ulcer and the healing rate. Table 3, figure 2 and 3 showed the antral and the duodenal bulb concentration of PGE2 before and after healing of the ulcer crater. As a group, tissue levels of PGE2, except for those of duodenal bulb PGE2 in the duodenal ulcer patients, were significantly increased when the ulcer was healed in both the gastric and the duodenal ulcer patients. Antral PGE2 levels in the active and healed stage of ulcer were 186.42┬▒70.51 vs 305.21┬▒104.91 (p<0.005) in the gastric ulcer patients and 204.94┬▒92.03 vs 271.02┬▒93.23 pg/mg. tissue (p<0.005) in the duodenal ulcer, respectively. The corresponding levels of the duodenal bulb were 79.44┬▒39.04 vs 128.84┬▒57.62 (p<0.005) and 99.66┬▒56.10 vs 112.60┬▒42.25 pg/mg. tissue (p<0.25). When the tissue PGE2 level were measured in same patients before and after the ulcer healing, both the antral and the duodenal PGE2 levels still showed significant difference between the active and the healed stage of ulcer (p<0.025 for GU and p<0.005 for DU) (Table 4 and Fig. 4). Although the difference in the bulb PGE2 in duodenal ulcer patients between different stages of ulcer seemed to be contradictory in each investigation, this might be resulted from the disparity of the study population in each group.
The present study shows that human antral and duodenal mucosa is capable of producing PGE2 and the antral PGE2 concentration was twofold more than those of duodenal bulb mucosa. This result agreed considerably with the antral levels reported by Ligumsky et al21) and the antral PGE2 concentration reported by Bennett et al22) in studies on surgical specimens. Konturek et al23) also reported that the oxyntic, antral and duodenal mucosa was capable of generating large amount of PGE2, and the antral mucosa was found to contain larger amount of prostaglandins than the other portion of stomach and duodenum. Similar result was also reported by Wright et al24) in 1982.
Both the antral and the duodenal PGE2 were significantly reduced in the active stage of ulcer disease and increased when the ulcer was healed. Mucosal PGE2 concentrations in the healing stage were similar to those of non-ulcer persons. Konturek et al23) found that healthy volunteers exhibited higher PGE2, although the difference in the generation of PGE2 between these two groups was not statistically significant. In their report, they suggested that PGE2 might be involved in the mechanism that protects the gastroduodenal mucosa against various kinds of damage. Wright et al24) also found that the significantly reduced mucosal level of PGE2 in patients with benign gastric ulcer and patients with healing ulcers had higher levels than those with non-healing ulcers but they suggested that the reduced level by itself was not directly responsible for the development of gastric ulcer disease but might be resulted from the varying degrees of the concommitant atrophic gastritis which was usually associated with gastric ulcer or gastric carcinoma. Ahlquist et al25) compared the duodenal prostaglandin synthesis activity between healthy volunteers and patients with duodenal ulcer disease and concluded that duodenal ulcer patients had a defect in post cibum duodenal prostaglandin production which was normally induced in healthy persons and this defective ŌĆ£physiologic adaptive cytoprotectionŌĆØ might be resulted either from the mucosal inflammation or from an intrinsic mucosal defect. They also pointed out that the duodenal ulcer did not appear to be due to an absolute deficiency of mucosal prostaglandin synthesis capacity and was not due to an abnormal synthesis profile. Hinsdale et al26) have reported that duodenal ulcer patients had lower concentrations of PGE in their plasma and basal gastric juice than had normal controls, and they suggested that prostaglandin deficiency might be an important etiological factor in peptic ulcer disease. Rachmilewitz et al27) and Sharon P et al28) found that cultured gastric and duodenal mucosa synthesized significantly less prostanoids in duodenal ulcer patients and they concluded that a decrease in endogenous prostanoid synthesis might have an important pathogenic role in peptic ulcer diseases. In contrast to these observations, Schlegel et al19) reported higher levels of the mucosal prostaglandin in gastric ulcer patients than normals and Tonnesen et al29), Cheung et al2), Ligumsky et al21), and Baker et al30) had failed to confirm this PGE deficiency in patients with duodenal ulcer.
In order to explain the exact pathogenic role of endogenous prostaglandins in the chronic peptic ulcer, the interrelationships between mucosal prostaglandins, gastric acid, gastric mucus, gastric and duodenal mucosal bicarbonate secretion, and influence on the mucosal blood flow and epithelial cell renewal must be clarified individually.
This study showed that patients with active ulcer had significantly lower levels of prostaglandins than non-ulcer patients and mucosal prostaglandins increased to the levels of non-ulcer patients when ulcers became healed. Considering these results, it could be concluded that prostaglandin deficiency in the antral and duodenal bulb mucosa may have an important pathogenic role in producing the chronic peptic ulcer disease.
Fig.┬Ā1.
Prostaglandin E2 levels in the antrum and duodenal bulb in patients with gastric and duodenal ulcer compared with non-ulcer persons. (The mean is indicated a bar). In non-ulcer persons the antral level is significantly higher than the duodenal bulb, p<0.005. The antral and duodenal bulb levels of PG E2 are significantly reduced both in the gastric ulcer patients (p<0.005 & 0.005) and duodenal ulcer patients (p<0.025 & 0.05).
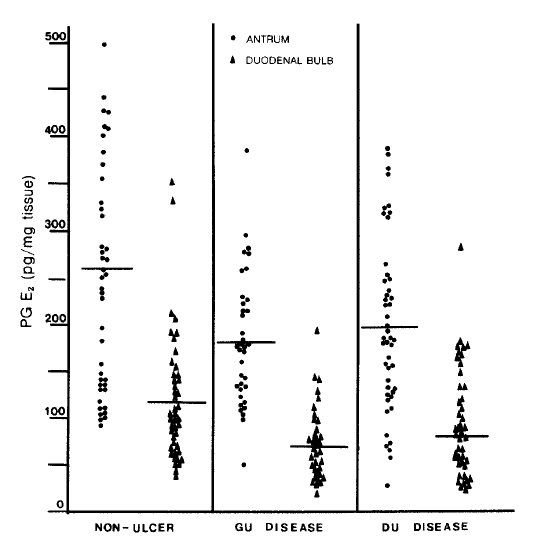
Fig.┬Ā2.
Mucosal prostaglandin E2 levels in patients with the gastric ulcer before and after their ulcers were healed. Both the antral and duodenal PG E2 are significantly increased when ulcers are healed. (antrum; p<0.005 duodenal bulb; p<0.005)
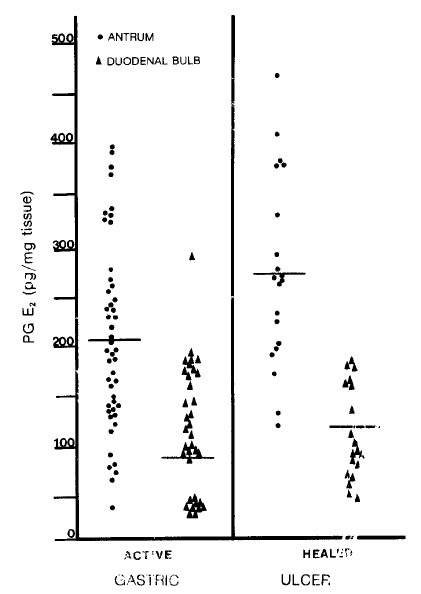
Fig.┬Ā3.
Mucosal prostaglandin E2 levels in patients with the duodenal ulcer before and after their ulcers are healed. Both the antral and duodenal bulb levels of PG E2 are increased when ulcers are healed (p<0.005 & p<0.25).
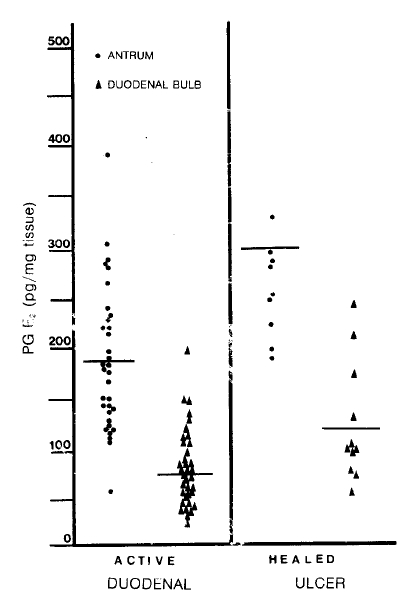
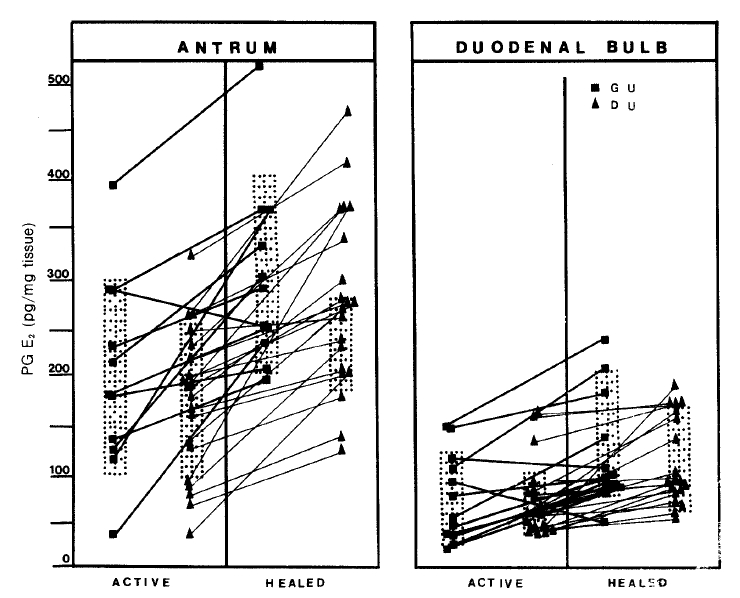
Fig.┬Ā4.
Mucosal prostaglandin E2 levels measured in same patients before and after their ulcers were healed. Antral and duodenal PG E2s are duodenal PG E2s are significantly increased in both groups. (GU; p<0.025, DU; p<0.005)
Table┬Ā1.
Subject Characteristics: Age, Sex, and Endoscopic Diagnosis
Table┬Ā2.
Mucosal Prostaglandin E2 levels in the Active Stage of Ulcers compared to Non-Ulcer persons(pg/mg. tissue)
|
Non-Ulcer (n=44) |
Active GU (n = 37) |
Active DU (n = 48) |
|
|---|---|---|---|
| Antrum | 258.17┬▒127.03 | 186.42┬▒70.51 | 204.94┬▒92.03 |
| Duodenal Bulb | 121.07┬▒67.46 | 79.44┬▒39.04 | 99.66┬▒56.10 |
Table┬Ā3.
Mucosal prostaglandin E2 levels in the Active and the Healed Stage of Ulcers (pg/mg. tissue)
Table┬Ā4.
Mucosal prostaglandin E2 levels measured in Same Patients Before and After Ulcer Healing (pg/mg. tissue)
REFERENCES
1. Bennett A, Stamford IF, Unger WG. Prostaglandin E2 and gastric acid secretion in man. J Physiol 229:349. 1973.



2. Cheung LY, Jubiz W, Moore JG. Gastric prostaglandin E output during basal and stimulated acid secretion in normal subjects and patients with duodenal ulcer. J Sur Res 20:369. 1976.

3. Wilson DE, PHilips C, Levine RA. Inhibition of gastric secretion in man by prostaglandin A2. Gastroenterology 61:201. 1971.


4. Carter DC, Karim SMM, Bhana D, Ganesan PA. Inhibition of human gastric secretion by prostaglandin. Brit J Surg 60:828. 1973.


5. Salmon JA, Karim SMM, Carter DC, Adaikan PG, Bhana D. Effect of 15 (R) 15-Methyl Prostglandin E2 methyl ester on basal and pentagastrin stimulated pepsin secretion in man. International Research Communications System, Medical Science 3:83. 1975.
6. Johansson C, Kollberg B. Stimulation by intragastrically administered E2 prostaglandins of human gastric mucus output. Eur J Clin Invest 9:229. 1979.


7. Feldman M. Gastric bicarbonate secretion in humans. Effect of pentagastrin, bethanechol, and 11, 16, 16-trimethyl prostaglandin E2. J Clin Invest 72:295. 1983.



8. Heylings JR, Feldman M. Stimulation of HCO3 secretion by the prostaglandin E2 analog enprostil: Studies in human stomach and rat duodenum. Prostaglandins 32:907. 1986.


9. Robert A, Schultz JR, Nezamis JE, Lancaster C. Gastric antisecretory and antiulcer properities of PGE2, 15-methyl PGE2, and 16, 16-dimethyl PGE2. Gastroenterology 70:359. 1976.


10. Kauffman GL, Grossman MI. Prostaglandin and cimetidine inhibit the formation of ulcers produced by parenteral salicylate. Gastroenterology 75:1099. 1978.


11. Fung WP, Karim SMM, Tye CY. Effect of 15 (R) 15-methyl prostaglandin E2 methyl ester on healing of gastric ulcers. Lancet 2:10. 1974.


12. Gibinsky K, Rybicka J, Mikos E, Nowak A. Double blind clinical trial on gastroduodenla ulcer healing with prostaglandin E2 analogues. Gut 18:636. 1977.



13. Rybicka J, Gibinsky K. Methyl-Prostaglandin E2 amaloguues for healing of gastro-duodenal ulcers. Scand J Gastroenterol 13:155. 1978.


14. Robert A. An intestinal disease produced experimentally by a prostaglandin deficiency. Gastroenterology 69:1045. 1975.


15. Konturek SJ, Piastucki I, Brzozowski T, Radecki T, Dembinska-Kiec A, Zmuda A, Gryglewski R. Role of prostaglandins in the formation of aspirin-induced gastric ulcers. Gastroenterology 80:4. 1981.


16. Robert A, Lancaster C, Hancher AJ, Nezamis JE. Mild irritants prevent gastric necrosis through prostaglandin formation: histologic study (abstr). Gastroenterology 74:1086. 1978.

17. Chaudhury TK, Robert A. Prevention by mild irritants of gastric necrosis produced in rats by sodium taurocholate. Dig Dis Sci 25:830. 1980.


18. Robert A. The role of prostaglandins in etiology and the treatment of gastroduodenal diseases. Proceedings of the Sixth International Congress of Pharmcology 5:161. 1975.
19. Schlegel W, Wenk K, Dollinger HC, Raptis S. Concetrations of prostaglandin A-, E- and F-like substances in gastric mucosa of normal subjects and of patients with various gastric diseases. Clin Sci Mol Med 52:255. 1977.

20. Baker R, Jaffe BM, Reed JD, Shaw B, Venables CW. Are prostaglandins deficient in peptic ulcerations ? Gut 18:950. 1977.
21. Ligumsky M, Sharon P, Karmeli F, Rachmilewitz D. Prostaglandins and the pathogenesis of duodenal ulcer: No correlation with the gastric mucosal PgE2 content. Isr J Med Sci 15:171. 1979.

22. Bennett A, Stamford IF, Stockley HL. Estimation and characterization of prostaglandins in the human gastrointestinal tract. Br J Pharmacol 61:579. 1977.



23. Konturek SJ, Obtulowicz W, Sito E, Olesky J, Wilkon S, Kiec-Dembinska A. Distribution of prostaglandins in gastric and duodenal mucosa of healthy patients: effects of aspirin and paracetamol. Gut 22:283. 1981.



24. Wright JP, Young GO, Klaff LJ, Weers LA, Price SK, Marks IN. Gastric prostaglandin E levels in patients with gastric ulcer disease and carcinoma. Gastroenterology 82:263. 1982.


25. Ahlquist DA, Dozois RR, Zinsmeister AR, Malagelada JR. Duodenal prostaglandin synthesis and acid load in health and in duodenal ulcer disease. Gastroenterology 85:522. 1983.


26. Hinsdale JG, Engel JJ, Wilson DE. Prostaglandin E in peptic ulcer disease. Prostaglandins 6:459. 1974.

27. Rachmilewitz D, Ligumski M, Fich A, Goldin E, Eliakim A, Karmeli F. Role of endogenous gastric prostanoids in the pathogenesis and therapy of duodenal ulcer. Gastroenterology 90:963. 1986.


28. Sharon P, Cohen F, Zifroni F, Karmeli F, Ligumski M, Rachmilewiz D. Prostanoid synthesis by cultured gastric and duodenal mucosa: Possible role in the pathogenesis of duodenal ulcer. Scand J Gastroenterol 18:1045. 1983.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



