 |
 |
| Korean J Intern Med > Volume 7(1); 1992 > Article |
|
Abstract
Background
Nucleation from supersaturated bile of calcium salts of cholesterol and bilirubinate is essential in the formation of gallstone. Nucleation requires gallbladder mucin and its main component, glycoprotein, may contribute to gallstone formation by providing a nidus or matrix for precipitation of lipid components. However, biliary protein patterns of patients with gallstones have not been completely explored.
Methods
We have tried to extract, isolate and characterize the proteins in patients with gallstones and without gallstones. 21 bile samples were obtained from patients with different types of gallstones and with no stones at cholecystectomy. Biliary protein concentrations were measured by Lowry and Bensadoun methods, and individual glycoproteins from each of the patients were compared by silver staining and densimetric quantification of Sodium Dodesyl Sulfate Polyacrylamide Gel Electrophoresis.
Results
1) Among 16 gallstones, 5 were cholesterol stones, 5 were calcium bilirubinate stones, and 6 were black pigment stones. 2) The mean protein concentration was highest in bile with cholesterol stones (47.6 mg/ml), 24.2 mg% in bile without gallstones, and 15.9 mg/ml in brown pigment stones. 3) Cholesterol gallstones were found to have 14.2 KD glycoproteins, whereas pigment stones were found to have 66 KD glycoproteins.
Biliary proteins have been suggested to play an important role in bile nucleation and gallstone formation. Supersaturation of gallbladder bile is the first step in gallstone formation1) and nucleation from supersaturated bile of calcium salts of cholesterol or bilirubinate have been implicated in gallstone formation2). Normal bile contains factors that inhibit nucleation, whereas lithogenic biles contain factors that promate nucleation3). Although the precise role of nucleation has not been well characterized, recent study suggest that these factors are probably proteins4ŌĆō6). It should be also suggested that nucleation requires an organic matrix, composed of small, soluble acidic regulatory proteins bound to a structural framework of high molecular weight gallbladder mucins.
Several investigators have reported results of measurements of total protein concentrations in biles of gallstone patients, but they differ in their opinions with different results7ŌĆō10). The idea that there might be a specific differences in the protein compositions in patients with cholesterol and pigment gallstones may provide information as to the nature of nucleating or antinucleating factors in bile. Little data are available, however, to compare the biliary protein patterns of patients with cholesterol or pigment stones and those without gallstones. We have tried to extract, isolate, and characterize the proteins in patients with gallstones and without gallstones.
Bile samples were obtained from patients with different types of gallstones (5 cholesterol, 5 brown pigment, and 6 black pigment) and 5 with no gallstones. Gallbladder bile was collected by needle puncture at cholecystectomy and stored frozen at ŌłÆ50┬░C under light protection.
Approximately 0.5 mg of dessicated powdered gallstone and 100 mg of KBr were jointly ground finely using motar and pestle. The mixture was redessicated for at least 1wk and then compressed in an evacuated stainless steel die at 22000 1b/in for 12 min. The resultant wafer was subjected immediately to infrared spectroscopy (Perkin-Elmer Corp.) Pigment content (calcium bilirubinate) was determined from absorbance at 1624 cmŌłÆ1, phosphate at 1030 cmŌłÆ1 and carbonate at 865 cmŌłÆ1 as described by Trotman et al11).
Proteins in bile were precipitated with trichloroacetate (TCA) and delipidated with ether/ethanol, sodium deoxycholate (10 ╬╝l) were added to the sample (200 ╬╝l), samples were then centrifugated at 1400 rpm 10 min, and the supernatant was aspirated and mixed with acetone. The mixture was centrifugated again at 1400 rpm for 10 min. and dried with nitrogen gas. Total protein concentrations were then measured using albumin as a standard by Lowry and Bensadoun technique12,13). Individual glycoproteins from each patient were compared by silver staining and densitometric quantification of SDS (Sodium Dodecyl Sulfate) Polyacrylamide Gel Electrophoresis14,15). Molecular weight and purity were determined by SDS-PAGE in 50 mM Tris-HC1, pH 8.3, containing 0.1% sodium dodesyl sulfate with a 5ŌĆō10% linear acrylamide gradient, developed at 25ŌĆō35mA for 4ŌĆō5hrs, and protein bands were identified by silver staining of the gel. Standard proteins used to determine molecular weight were as follows; phospholipase (97K), bovine serum albumin (66K), ovalbumin (45K), glyceraldehyde-3-phosphate dehydrogenase (36K), carbonic anhydrase (29K), trypsin inhibitor (29K), a-lactalbumin (14.2K), and myoglobin digestes (8K, 6K).
Three groups of patients were studied and the characteristics of the patients are summarized in Table 1. The three study groups were similar with respect to age, sex, laboratory studies, risk factors, etc. The incidence of bacteria was O in bile with black pigment stones, 20% with cholesterol stone and 100% with brown pigment stones. Most frequently isolated bacterial species was E. coli (50%), and others were pseudomonas aeruginosa, Citrobacter freundii and Clostridium baratii.
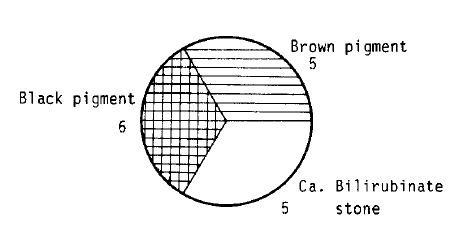
As determined from infrared spectra, the three types of gallstones could be classified according to their compositions. Among 16 gallstones, 5(31%) were cholesterol stones, 5 (31%) were calcium bilirubinate stones, and 6 (38%) were black pigment stones (Fig. 1). Pigment accounted more than 50% of pigment gallstone. Calcium for phosphate and carbonate were detectable in about 80% of black pigment stones in varing concentrations, and the levels were significantly lower in brown pigment stones than in black pigment stones. Calcium bilirubinate was present in all brown pigment stones and represents a greater proportion than in black pigment stones.
The protein yield of repeated extraction was not statistically different in three types of gallstones. The mean protein concentration was highest in bile with cholesterol gallstones (47.6┬▒25.9 mg/ml; 19ŌĆō75), 24.2┬▒17.3 mg/ml in bile with black pigment stones (10ŌĆō51), 16.5┬▒4.4 mg/ml in bile without stones (11ŌĆō20), and the concentration was lowest in bile with brown pigment stones (15.9┬▒18.9 mg/ml; 7.3ŌĆō51) (Fig. 2).
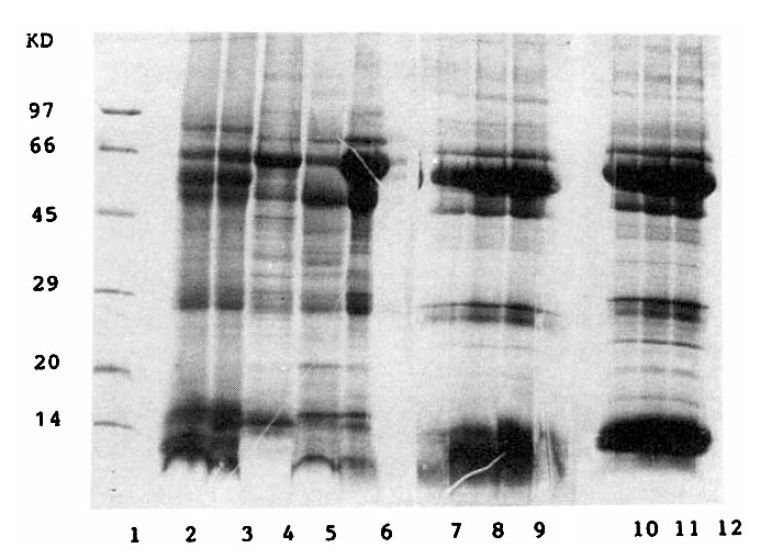
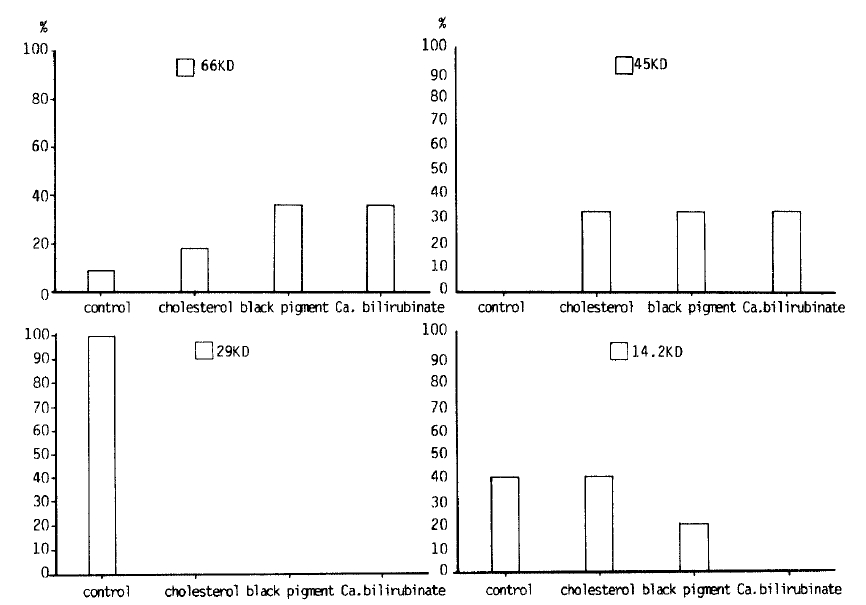
Fig. 3 shows the protein patterns on a SDS-polyacrylamide gel. Proteins from the stones consisted largely of major bands below 66 KD. Left first lane shows molecular weight protein standards and the other lanes show several different protein fractions of 3 different gallstones. Of the glycoproteins identified from SDS-PAGE, differences among groups were noted in the bands at 66000, 45000, and 14200 daltons. (Fig. 4) Cholesterol gallstones were found to have 14.2KD glycoprotein and brown and black pigment stones were found to have 66KD glycoproteins. Patients without gallstones had a 29KD glycoprotein, whereas patients with gallstones did not have any 29KD glycoprotein. All patients with gallstones had 45KD glycoproteins, whease patients without gallstones did not have 45KD glycoprotein.
Gallstones have become a major public health problem in all parts of the world16), and the incidence has been increasing in the Orient, even in our country17,18). There are two major classes of gallstones: cholesterol gallstones and pigment gallstones on the basis of their major composition, characteristics and pathogenesis19). Cholesterol stones consist of at least 70% cholesterol, often with alternating layers of brown to black pigments, in which case they are called mixed stones20). Pigment stones are subclassified into two groups: black pigment stones and brown pigment stones21). Black pigment stones contain much insoluble black pigment that is highly crosslinked network polymer or tetrapyrroles22), which is mixed with calcium salts of bilirubinate, carbonate and/or phosphate11,21). Brown pigment stones consist mainly of the insoluble calcium bilirubinates, calcium soaps of fatty acids, and a significant proportion of cholesterol 23,24).
The classification of gallstones has been based on morphological and chemical analysis but has been limited by the variety and insolubility of many of the components24). The introduction of solid state analytical techniques such as X-ray diffraction and infrared spectroscopy has permitted identification of insoluble components of gallstones25,26,27). The infrared spectroscopy has an advantage that it could identify the components in pigments stones without requiring gallstones crystallinity and solubilization22,23,28).
Gallstone pathogenesis can be broken down into three distinct stages: 1) supersaturation, 2) nucleation, and 3) stone growth. Early investigators focussed primarily on cholesterol saturation, and most studies revealed that normal bile may be supersaturated and pathological bile is always supersaturated. However, supersaturation is not a sufficient condition for nucleation and gallstone formation7,29,30). Cholesterol monohydrate crystals precipitate much more rapidly from lithogenic biles than from normal biles, due to potent factors that promote nucleation of cholesterol31). Normal bile also contains potent proteins that inhibit nucleation of cholesterol and calcium salts from supersaturated solutions including abnormal biles3,5,32). These inhibitory proteins are diminished in the gallbladder bile of patients with gallstones33,34). A great deal of interest has recently focused on the identification of the potential pronucleating factors.
In the present experiment, there was no statistical difference in protein composition among three types of gallstones. Harvey et al also reported that the glycoprotein concentrations in three types of gallstones were not significantly different35). The mean protein concentrations in our study were a little bit higher than those from Harvey's study and those determined by Lowry and Bradford36). However, the mean protein concentration was higher (47.6┬▒25.9 mg/ml) in cholesterol gallstones than in pigment stones or no stones, and it is still possible that the onset of nucleation may be associated with a temporary increase in protein concentration which then returns to normal levels once nucleation has occurred.
As in most biomineralization processes in mammals, there should be alterations of the biomineralization system in cholelithiasis: 1) alterations of mucin, 2) alterations in nucleating proteins, and 3) alterations in antinucleating proteins35).
Marked increase in the concentration of cholesterol-nucleating protein has been found in the bile from patients with cholesterol gallstones. This protein, however, is not shown to be increased in concentration in the bile of pigment gallstones. We agree with StrasbergŌĆÖs groups that there should be an alteration in protein in lithogenic bile, and these proteins may be nucleated cholesterol or calcium bilirubinate from gallbladder bile6).
The present result is similar to our previous results which were done with extraction of gallstones38). We still need to revise the extraction method to get more protein.
We try to determine the molecular weight and purity by SDS-PAGE electrophoresis, and the protein bands could be identified by silver staining of the gel. SDS-PAGE showed major bands at approximate molecular weight 66, 45, 29, and 14.2 KDa. The lowest molecular weight bands stained heavily, indicating many protein residues39). Cholesterol gallstones were found to have 14.2 KDa glycoproteins, whereas pigment stones had 66 KDa glycoproteins. All patients with gallstones had 45 KDa glycoproteins, whereas patients without gallstones didnŌĆÖt. These results suggest that some of these proteins may serve as nucleating agents. The present result differs from the 7 KDa bile-proteins isolated from T-tube bile by Etter-Kjelsaas and Kuenzle40). The present study, however, would appear to confirm the result of Shimizu et al. with both experiments suggesting that both cholesterol and pigment gallstones contain acidic proteins with many phosphorylated residues41). They thus resemble the low molecular weight proteins from normally calcifying tissues (eg. phosphorylations in dental enamels and osteocalain from bone), that regulate the biomineralization processes by binding calcium salts39). They are generally small glycoproteins, which are acidic due to a high content of aspartate, glutamate, phosphorylated/sulfated serine/threonin, and often gamma-carboxyglutamate residues42,43). Pancreatic and urinary calculi were also found to contain similar proteins44,45).
We thought that the acidic phosphorylated proteins from gallstones may play a similarly important role in the initiation of nucleation process. However, it remains to be determined whether the protein functions as a promoter or inhibitor of nucleation. Moreover, it is known that the same protein can act as nucleating or antinucleating factors at different concentrations, or under different conditions46).
We propose that lithogenic bile may induce an increase in gallbladder protein secretion and gallbladder proteins from both cholesterol and pigment stones could play an important role in the nucleation and growth of the calcium salt crystals. Further studies are necessary to purify and characterize the biliary proteins.
Fig.┬Ā3.
SDS-PAGE of proteins from different types of gallstones. Lane 1, low molecular weight protein standards; land 2ŌĆō6, protein fractions of cholesterol stones; lane 7ŌĆō9, protein fractions of brown pigment stones; lane 10ŌĆō12, protein fractions of black pigment stones.
Table┬Ā1.
PatientsŌĆÖ Characteristics
REFERENCES
1. Admirand WH, Small DM. The physiochemical basis of cholesterol gallstone formation in man. J Clin Invest 47:1043. 1968.



2. Holtzbach RT. Recent progress in understanding cholesterol crystal nucleation as a precursor to human gallstone formation. Hepatology 6:1403. 1986.


3. Holtzbach RT, Kibe A, Thiel E, Howell JH, Marsh M, Hermann RE. Biliary proteins. Unique inhibitors of cholesterol crystal nucleation in human gallbladder bile. J Clin Invest 73:35. 1984.



4. Lee SP, LaMont JT, Carey MC. Role of gallbladder mucus hypersecretion in the evolution of cholesterol gallstones: studies in prairie dog. J Clin Invest 67:1712. 1981.



5. Kibe AR, holtzbach RT, Larusso NF, Mao SJT. Inhibition of cholesterol crystal formation by apolipoproteins in supersaturated model bile. Science 225:514. 1984.


6. Gallinger S, Harvey PRC, Petrunka CN, IIson RG, Strasberg SM. Biliary proteins and the nucleation defect in cholesterol cholelithiasis. Gastroenterology 92:867. 1987.


7. Holan KR, Holtzbach RT, Heerman RT, Cooperman AM, Claffey WJ. Nucleation time; a key factor in the pathogenesis of cholesterol gallstone disease. Gastroenterology 77:611. 1979.


8. Sewell SB, Mao SJT, Kawamoto T, Larusso NF. Apolipoproteins of high, low, and very low density lipoproteins in human bile. J Lipid Res 24:391. 1983.


10. Arianoff AA, Heremans J. Les proteins dans la bile humaine normle et pathologiique. Etude quantitative Acta Gastro-Enterol Belg 30:387. 1967.
11. Trottman BW, Morris TA, Sanchez HM. Pigment vs cholesterol cholelithiasis: identification and quantification by infrared spectroscopy. Gastroenterology 72:495. 1977.


12. Lowry OH, Rosenbrough NJ, Fan AL, Randall RJ. Protein measurement with the folen phenol reagent. J Biol Chem 193:263. 1951.
13. Bensadoun A, Weinstein D. Anal Biochem 70:241. 1976.
14. Green MR, Pastewka JV, Peacock AC. Differential staining of phosphoproteins on polyacrylamide gels with a cationic carbocyanine dye. anal Biochem 56:43. 1973.


15. Hancock R, Wray VP, Boulikas T, Wray W. Silver staining of proteins in polyacrylamide gels. Anal Biochem 118:197. 1981.


16. Sutor DJ, Wolley SE. A statistical survey of composition of gallstones in eight countries. Gut 12:55. 1971.



17. Nagase M, Tanimura H, Motoichi S, Hikasa Y. Present features of gallstones in Japan. Am J Surg 135:788. 1978.


18. Kim BR, Sho EW. Characteristics and changes of gallstone in Korea. J Kor Med Asso 31(1):23. 1988.
20. Trottman BW, Ostrow JD, Soloway RD. Pigment vs cholesterol cholelithiasis: comparison of stone and bile composition. Am J Dig 19:585. 1974.

22. Rege RV, Carr SH, Ostrow JD, Ohkubo H. Polymernetworks in pigment and cholesterol gallstones assessed by equilibrium swelling and infrared spectroscopy. Gastroenterology 87:805. 1984.


23. Soloway RD, Malet PE, Dabezies MA, Huang G, Long WB, Gadacz TR. Quantitative infrared spectroscopy of common bile duct gallstones. Gastroenterology 94:1217. 1988.


24. Nakayama F. Quantitative microanalysis of gallstones. J Lab Clin Med 72:629. 1969.
25. Sutor DJ, Wolley SE. X-ray diffraction studies of the composition of gallstones from English and Australian patients. Gut 10:681. 1969.



26. Choi SG, Kim JW, Choi YK. Identification and quantification of gallstones by infrared spectroscopy. Kor J Int Med 29(3):299. 1985.
27. Bogren H, Larsson K. Crystalline composition of biliary calculi. Scand J Clin Lab Invest 15:457. 1963.


28. Sutor DJ, Wilkie LI. The crystal salts of calcium bilirubinate in human gallstones. Clin Sci Molec Med 53:101. 1977.
29. Holtzbach RT, Marsh M, Olszewski M, holen KR. Cholesterol solubility in bile: Evidence that supersaturated bile is frequent in healthy man. J Clin Invest 52:1467. 1973.



30. Sedaght A, Grundy SM. Cholesterol crystals and the formation of cholesterol gallstones. N Eng J Med 302:1224. 1980.

32. Swaretta , Ligabue , Garuti , et al. Inhibitory activity of gallbladder bile on calcium carbonate crystallization in Vitro. Scand J Gastroenterol 19:628. 1984.

33. Sutor , Percival . The effect of bile on the crystallization of calcium carbonate, a constituent of gallstones. Clin Chim Acta 89:479. 1978.


34. Knyrim , Vakyl N. Bile from patients with gallstones is defective in an antinucleating factor for calcium carbonate. Gastroenterology 96:614. (abstr):1989.
35. Harvey PRC, Rupar CA, Gallinger S, Petrunka CN, Strasberg SM. Quantitative and qualitative comparison of gallbladder mucus glycoprotein from patients with and without gallstones. Gut 27:374. 1986.



36. Harvey PRC, Upadhya GA, Toth JL, Strasberg SM. Flurometric assay of protein in native human bile. Chim Acta 183:147. 1989.

37. Strasberg SM, Taylor RD, Petrunka CN, Ilson RG, Burnstein MJ. Evidence for a potent nucleating factors in the gallbladder bile of patients with cholesterol gallstones. Gastroenterology 85:801. 1983.


38. Yang CS, Han DS, Hahm JS, et al. A study for the significance of gallstone proteins in the nucleation of the gallstones. Korean J Int Med 22(2):373. 1990.
39. Veis A, Sabsay B, Rahima M, Clohisy J, Albinger TM, Veis DJ. Matrix proteins of the teeth of sea Urchin Lytechinus Variegatus. J Experm Zoology 240:35. 1986.

40. Etter-Kjelsaas H, Kuenzle CC. A polypeptide conjugate of bilirubin from human bile. Biochim Biophys Acta 400:83. 1975.


41. Shimizu S, Sabsay B, Veis A, Ostrow JD, Rege RV, Dawes LG. Isolation of an acidic protein from cholesterol gallstones which inhibits the precipitation of calcium carbonate in vitro. J Clin Invest 84:1990. 1989.



42. Veis A, Tsay TG. Preparation, detection and characterization of an antibody to rat alpha-phosphophorin. Biochemistry 24:6363. 1985.


43. Hauschka PV. Quantitative determination of ╬│-carboxyglutamic acid in proteins. Analytical Biochemistry 80:212. 1977.


44. Caro ADE, Multigner L, Lafont H, Lambardo D, Sarles H. The molecular characteristics of human pancreatic acidic phosphoprotein that inhibits calcium carbonate crystal growth. J Bioch 222:669. 1984.







 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



