INTRODUCTION
IgA nephropathy is one of the most common primary glomerulonephritis in the world, including Korea1ŌĆō4). The main histologic feature is the mesangial cell proliferation, which suggests the role of cytokines and growth factors in its pathogenesis5). Cytokines and growth factors are known to be derived from intrinsic glomerular cells and/or infiltrating inflammatory cells5). Among the infiltrating cells, monocytes, which are known to be the principal effectors cells in immune mediated nephritis, frequently infiltrate the glomerular mesangium6), and their cytokines are revealed to stimulate the mesangial cell proliferation. Among them, interleu-kin-1 (IL-1), tumor necrosis factor ╬▒ (TNF ╬▒) and interleukin-6 (IL-6) are primarily released by activated monocytes/macrophages5,7).
To investigate the role of mononuclear cells and their products on rat mesangial cell proliferation, we evaluated the effect of mitogen-stimulated PBMC culture supernatant of patients with IgA nephropathy on rat mesangial cell 3[H]-thymidine uptake and measured the concentration of cytokines in the supernatant to know which cytokine in PBMC culture supernatant has a major influence on mesangial cell 3[H]-thymidine uptake.
ATERIALS AND METHODS
1. Subjects
PBMC were isolated and cultured from ten patients with primary IgA nephropathy and from ten normal controls. The clinical and laboratory characteristics of the patients whose PBMC were isolated and cultured were shown in Table 1. The diagnosis of IgA nephropathy was based on the presence of predominant mesangial immunofluorescent IgA deposits and mesangial and paramesangial electron-dense deposits in renal biopsy specimens. Patients with the clinical and laboratory evidences of hepatic disease, systemic lupus erythematosus, Henoch-schoenlein nephritis and other systemic diseases were excluded. No patient had renal insufficiency or nephritic syndrome and all patients were in clinically quiescent state without fever, gross hematuria and upper respiratory or gastrointestinal illness. No Patients had infection or was on corticosteroid or immunosuppressive treatment within at least on month or entry into the study.
Medical doctors and nurses, matched for age and sex with the patients with IgA nephropathy, were selected as normal controls.
2. Preparation of PBMC Culture Supernatant
PBMC were isolated by Ficoll-Hypaque density gradient method8) from heparinized venous blood in patients with IgA nephropathy and healthy controls. The cell suspension recovered at the interface was resuspended in RPMI 1640 at a concentration of 5├Ś106 cells/ml. The cells were stimulated with phytohemaglutinin (20 ╬╝g/ml) and concanavalin A (10 ╬╝g/ml). The PBMC were cultured at 37┬░C in a 5% CO2 for 24 hours. After 24 hours, the supernatant was collected and stored at ŌłÆ70┬░C until tested.
3. Preparation of Mesangial Cell Culture
Kidneys from Spragde-Dawley rats weighing 100 to 150 g were removed. The cortex was minced into small pieces and passed through a series of steel sieves. The glomeruli collected on the top of the 75 ╬╝m sieve were rinsed with HBSS and were digested with 1 ml type IV collagense (GIBCO, NY, USA) at 37┬░C for 30 minutes with shaking. After centrifugation, the pellet was suspended in DMEM and plated into a tissue culture flask containing DMEM supplemented with 17% fetal calf serum (FCS), 100 U/ml penicillin, 100 ╬╝g/ml streptomycin, 10╬╝g/ml insulin and L-glutamine. After three or four passages, no endothelial or epithelial cells were seen and the cultures consisted exclusively of mesangial cells. The mesangial cells were characterized by using phase contrast microscopy and the cells had prominent intracellular myosin fibrils and were negative with antibodies (Becton Dickinson, Mountain View, CA, USA) to common leukocyte antigen and Factor VIII by immunofluorescent staining9). The cells were capable of growth in D-valine substituted medium and were not sensitive to puromycin.
4. Assay for Mesangial Cell 3[H]-thymidine Uptake
The number of mesangial cells was adjusted into 105 cells/ml in DMEM containing 15% FCS and 10 ╬╝g/ml insulin and plated into 96 well microplate at a concentration of 104 cells/well. After culture in a 5% CO2 at 37┬░C for 48 hours, the medium was changed with DMEM containing diluted (1:2 or 1:10) PBMC culture supernatant and incubated for 24 hours. 1 ╬╝Ci of 3[H]-thymidine (New England Nuclear, MA, USA) was pulsed to each well and the culture was incubated for 18 hours. After washing the cells with PBS, the cells were harvested and the radioactivity of incorporated 3[H]-thymidine was counted under ╬│-scintillation counter (Beckman 800, Irvine, CA, USA).
5. Lymphocyte Subsets and Natural Killer (NK) Cell Activity Measurement
Assay for the lymphocyte subsets was done by the method of indirect immunofluorescence (IF) using monoclonal antibodies (Becton Dickinson, CA, USA). 100 ╬╝l of the lymphocyte suspension adjusted to 5├Ś106 cells/ml were placed in plastic tubes (Costar 2022, MA, USA) and incubated with 20 ╬╝l of anti-leu-2a, anti-leu-3a, anti-leu-4, and anti-leu-12 for 30 minutes at 4┬░C respectively. After incubation, the cells were washed twice in PBS and labeled with 100 ╬╝l of mounting medium (PBS, pH 7. 4, containing 30% glycerol) was added to cell suspension and one drop was examined under fluorescence microscope (Olympus, type BH-2, Japan).
NK cell activity was assayed by a chromium-release method of Jondal et al10). K-562 cells derived from a patient with chronic myeloid leukemia were used as target cells. Target cells were suspended at a concentration of 5├Ś106 cells/ml in complete culture medium and 100 ╬╝Ci of Na251CrO4 (New England Nuclear, MA, USA) was added to the 0.5 ml of cell suspension. After incubation for 90 minutes at 37┬░C in a 5% CO2 in air, cells were washed three times with culture medium and 10 ╬╝l of cell suspension adjusted to 5├Ś106 cells/ml in complete culture medium were placed into 96-well microplate (Costar 3799, MA, USA). 200 ╬╝l of effectors peripheral blood lymphocyte adjusted to the concentration of 1├Ś106 cell/ml were added to target cells in triplicate with the ratio of lymphocyte to target cell of 40:1. Target cells were incubated in complete culture medium as controls for spontaneous release (SR), and maximum release (MR) was measured after the addition of 1% Triton X-100 solutions. Samples were counted in a gamma-counter (Gamma 5500; Beckman, CA, USA). Percent cytotoxicity was calculated with the previous formula52).
RESULTS
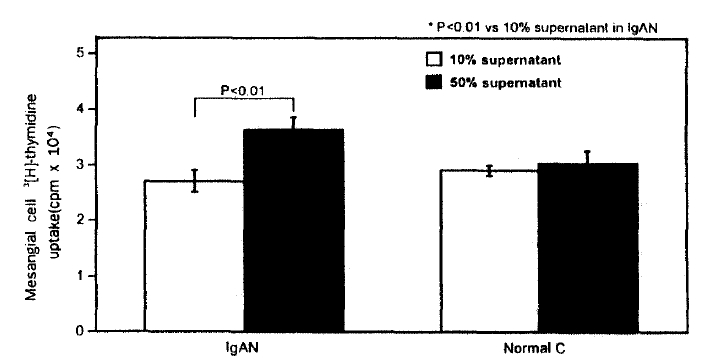
1. Effect of PBMC Culture Supernatant on Rat Mesangial Cell 3[H]-thymidine Uptake
The PBMC culture supernatant of the patients with IgA nephropathy induced the higher 3[H]-thymidine uptake into mesangial cell (37297┬▒2421 cpm) compared with that of normal controls (30957┬▒2469 cpm) when 1:2 diluted PBMC culture supernatant of the patients with IgA nephropathy and normal controls was added to the mesangial cell culture medium (Table 2, Fig. 1). However, the addition of 1:10 diluted supernatant into mesangial cell culture didnŌĆÖt show any difference in 3[H]-thymidine uptake between IgA nephropathy and normal controls (Table 2, Fig. 1). The uptake of 3[H]-thymidine into mesangial cell induced by 1:2 diluted PBMC culture supernatant was significantly higher than that induced by 1:10 diluted supernatant in IgA nephropathy (37297┬▒2421 cpm vs 27459┬▒2025 cpm, P=0.0076). This difference, however, was not observed in normal controls (Table 2, Fig.1).
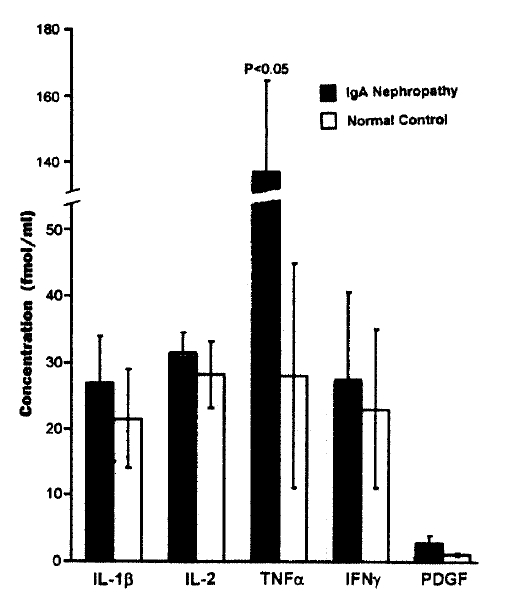
2. The Concentration of Cytokines in PBMC Culture Supernatant
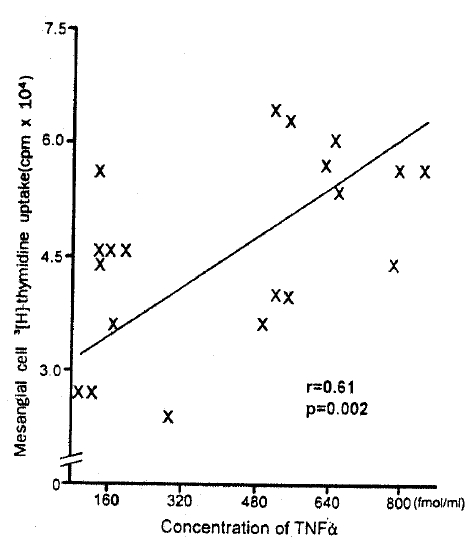
The concentration of TNF ╬▒ in PBMC culture supernatant of patients with IgA nephropathy was significantly higher than that of normal controls (136.1┬▒35.0 fmol/L vs 28.2┬▒19.0 fmol/L, p<0.05). Moreover, the concentration of TNF ╬▒ was significantly correlated with the mesangial cell 3[H]-thymidine uptake in patients with IgA nephropathy (r=0.61, p = 0.002) (Fig. 3). The concentration of IL -1╬▓, IL-2, IFN-╬│, and PDGF was higher in PBMC culture supernatant of patients with IgA nephropathy compared with that of normal controls. However, no statistical significance was observed (Fig. 2).
3. Lymphocyte Subsets and NK Cell Activity
When the lymphocyte subsets in patients with IgA nephropathy were compared with that in normal controls, the percentages of CD3 and CD8 cell were significantly lower in patients with IgA nephropathy than those in normal controls, and the percentages of B cells and NK cell activity were significantly higher in patients with IgA nephropathy than those in normal controls (Table 3). Because the percentages of CD3 cell were low in patients with IgA nephropathy, the CD4/CD8 ratio was significantly higher in patients with IgA nephropathy compared with normal controls (Table 3). There was no correlation between the changes of the lymphocyte subsets or NK cell activity and the mesangial cell 3[H]-thymidine uptake or the cytokine concentration including TNF ╬▒ in PBMC culture supernatant (data not shown).
DISCUSSION
Since JonesŌĆÖ first report that monocytes/macrophages might be involved in the pathogenesis of glomerulonephritis9), many immunocytochemical, enzyme cytochemical, and electron microscopic studies have been performed to identify the monocytes/macrophages in the glomeruli of various types of human and experimental glomerulonephritis10ŌĆō15). The reports for occurrence of monocytes/macrophages in the glomeruli of IgA nephropathy were variable, perhaps because of the difficulty in detecting monocytes/macrophages in the tissue10ŌĆō15). Arima et al6) examined a specimen of renal biopsy by the indirect immunoperoxidase method, using anti-human monoclonal antibodies, and by ultrastructural peroxidase cytochemistry to clarify the significance of mononuclear phagocytes in IgA nephropathy, and suggested that mononuclear phagocytes might play an important role in the pathogenesis of mesangial hypercellularity in IgA nephropathy. The mononuclear cell has been known as one of the principal effectors cells leading to regulation of various cellular functions by infiltrating the glomerular mesangium and secreting various cytokines including IL-1, IL-6, PDGF and TNF ╬▒ in immune mediated nephritis5,7,16,19). it was reported that antibodies directed against monocytes might have a beneficial effect on human nephritis18). The mesangial cell proliferation might be affected by inflammatory cytokines, such as IL-1, TNF ╬▒ and IL-6, secreted from infiltrating mononuclear cells or from intrinsic glomerular cells in IgA nephropathy. However, there has been no consistency about the mitogenic or antimitogenic activity of TNF ╬▒ on the mesangial cell in vitro as well as in vivo.
The results of the present study that mitogen stimulated PBMC culture supernatant of IgA nephropathy induce more 3[H]-thymidine into mesangial cell than that of normal controls, and that dilution of supernatant decreased the 3[H]-thymidine uptake into mesangial cell in IgA nephropathy suggest that the mononuclear cells of IgA nephropathy may have an intrinsic property to produce more cytokines which are mitogenic to mesangial cells and/or to produce less antimitogenic cytokines than that of normal controls. This is consistent with the previous report that the inflammatory cell, such as monocyte or macrophage, may infiltrate the glomerulus in response to inflammation or infection, and that its secreted products act as a mitogen to mesangial cell19).
In the animal model of nephritis, TNF ╬▒ can exacerbate proteinuria and accelerate renal disease20ŌĆō21). TNF ╬▒ is known to induce changes in renal endothelial and mesangial cells22,23), induce the expression of major histocompatibility complex class I, II and intercellular adhesion molecule-124,25) and induce the release of other potentially harmful cytokines26) or vasoactive factors27) that may increase the cytotoxic potential of resident glomerular macrophages. The production of TNF ╬▒ may be primarily from the resident monocyte/macrophage and the glomerular mesangial cell, and such production has been postulated to play a role in glomerular damage28ŌĆō32).
The amount of TNF ╬▒ mRNA in PBMC from patients with periarteritis nodosa and WegenerŌĆÖs granulomatosis was reported to be increased compared with healthy subjects33). PBMC from patients with idiopathic nephritic syndrome in relapse also produced higher amounts of TNF ╬▒ than those from healthy control children, and patients in remission or being treated with prednisone or cyclosporine had a TNF ╬▒ production similar to or only slightly higher than controls34) Recently, in one study performed to investigate the role of cytokines in the mediation of glomerular injury in the nephritic syndrome caused by minimal change nephropathy, focal and segmental glomerulosclerosis and membranous nephropathy, mitogen-stimulated PBMC of nephritic subjects released an excess TNF ╬▒ compared with controls, a response not consistently observed for IL-1 ╬▓, IL-2, IFN-╬▒, and IFN-╬│35).
For the effects of TNF ╬▒ on mesangial cells, reports have provided conflicting results. Yamamoto et al36) suggested that TNF ╬▒ augmented the proliferation of rat mesangial cells in the presence of 5% fetal bovine serum. By contrast, Floege et al5) suggested that TNF ╬▒ had growth inhibitory activity. Most of these equivocal results are likely to arise from differences of species or culture condition5). It is suggested that the mitogenic effect of TNF ╬▒ was low-grade when compared with other growth factors such as PDGF and was only observed at relatively high concentrations of the stimulus5).
This study shows that supernatant from mitogen-stimulated PBMC of patients with IgA nephropathy contains higher concentration of TNF ╬▒ compared with normal controls and that there was good correlation between the concentration of TNF ╬▒ in supernatants and the 3[H]-thymidine uptake of mesangial cells. The result of this study is consistent with that of Yamamoto et al and suggests that the TNF ╬▒ secreted from mitogen-stimulated PBMC may be the stimulatory factor for the mesangial cells.
Recent study on the effects of environmental lections on some cytokines synthesis in cultured rat mesangial cells showed that TNF release was maximally enhanced in comparison to untreated cells with ConA at the concentration of 100 ╬╝g/ml37). In this study, PBMC were stimulated with the ConA at the concentration of 10 ╬╝g/ml and the PHA of 20 ╬╝g/ml. although the concentration of TNF ╬▒ in the supernatants from PBMC was not measured in the absence of these mitogens, and less amount of ConA was added to the PBMC culture. ConA and/or PHA might have an influence on the elevated concentration of TNF ╬▒ in the supernatant.
PDGF has been known as one of the most potent mesangial cell mitogens4). In this study, the concentration of PDGF in PBMC culture supernatant of IgA nephropathy was higher than that of normal controls. However, the difference of the concentration between them was not statistically significant. So, the role of PDGF on the increased 3[H]-thymidine uptake cannot be determined in this study. IL-2 has an autocrine function for the T cells and is suggested to play some roles in the pathogenesis of immunological disorder38,44). However, it is uncertain whether this cytokine is mitogenic for mesangial cells or not. Authors previously observed that IL-2 activity of PHA-stimulated PBMC culture supernatant in IgA nephropathy was higher than that of normal controls44). In this study, the IL-2 concentration measured by radioimmunoassay in PBMC culture supernatant in IgA nephropathy was not higher than that in normal controls. Taken together, itŌĆÖs suggested that IL-2 may not be the main factor for the mesangial cell proliferation. It is known that IFN-╬│ is an immune regulating factor against viral infection. Because some patients with IgA nephropathy have unspecified viral infection of the upper respiratory or gastrointestinal tract, it can be said that IFN-╬│ may be related to the pathogenesis of IgA nephropathy. However, we did not find the increased production of IFN-╬│ by PBMC from IgA nephropathy.
Studies on the T cell subsets in peripheral blood of the patients with IgA nephroapthy have reported somewhat contradictory results. Some reports have showed the increased percentages of CD4 cells and CD4/CD8 ratio as well as the reduced percentages of CD839,40), while no abnormalities in the percentages of CD4 cells, CD8 cells and CD4/CD8 ratio were observed by others41,42). This study shows the reduced percentages of CD8 as well as the increased percentages of CD4/CD8 ratio. These results suggest that defective immune suppression caused by the decreased activity of CD8 cell may be related to the pathogenesis of IgA nephropathy.
It has been proposed that NK cells play an important role in surveillance mechanisms postulated to provide resistance against tumor and viral infections43). Because there is a close temporal relationship between the onset of IgA nephropathy and the upper respiratory infection in IgA nephropathy, the measurement of NK cell activity in this disease may be somewhat interesting. Our previous study showed that NK cell activity of the patients with IgA nephropathy was significantly higher than that of healthy controls44), and the result of this study was consistent with the previous finding.
In conclusion, these data suggested that mononuclear cell of IgA nephropathy may have the intrinsic property to produce more TNF ╬▒, when stimulated by mitogen, which might be related to the mesangial cell proliferation in IgA nephropathy, and the potential role of PBMC as active producers of cytokines relevant for mesangial cell proliferation.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





