INTRODUCTION
Human parvovirus (HPV) B19 is an encapsulated, minute virus with a single-stranded DNA genome that belongs to the Parvoviridae family1). Several types of parvovirus cause disease in some animals, but HPV B19 is the only parvovirus which can cause disease in humans. HPV B19 is associated with several clinical syndromes, ranging from subclinical infection to chronic infection or severe hemophagocytic syndrome in immunocompromised patients. HPV B19 has been identified as the cause of erythema infectiosum (fifth disease) in children2), and fetal hydrops and spontaneous abortion during pregnancy3). This virus also causes acute arthritis and reactive arthropathy in adults4).
The association of HPV B19 with aplastic crises in carriers of hereditary hematologic abnormalities is well-documented5). Most reported cases have been limited to patients with sickle cell disease. This association has also been described, although much less frequently, in patients with hereditary spherocytosis (HS)6-8). Most of the affected patients were children or adolescents with HS which had already been documented prior to aplastic crisis. As in the case of our patient, however, such crisis may be the initial presentation of HS9). To the best of our knowledge, this is the first report in Korea which describes an adult with aplastic crisis induced by HPV B19 as an initial presentation of HS.
CASE REPORT
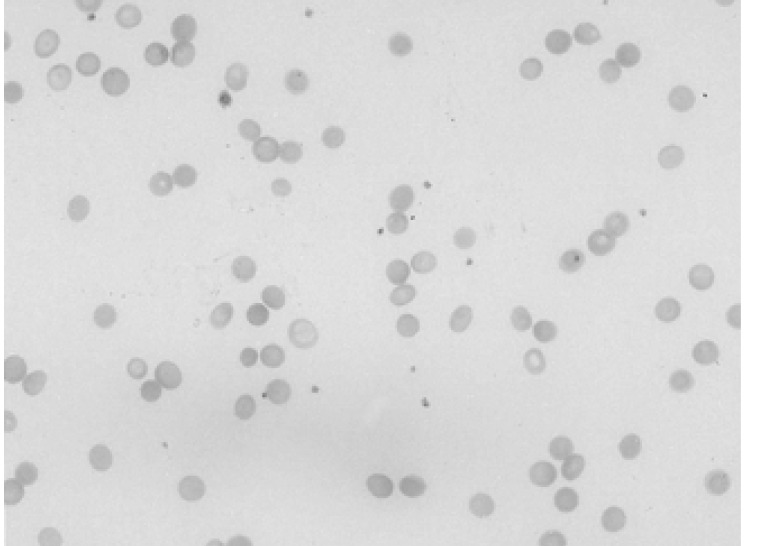
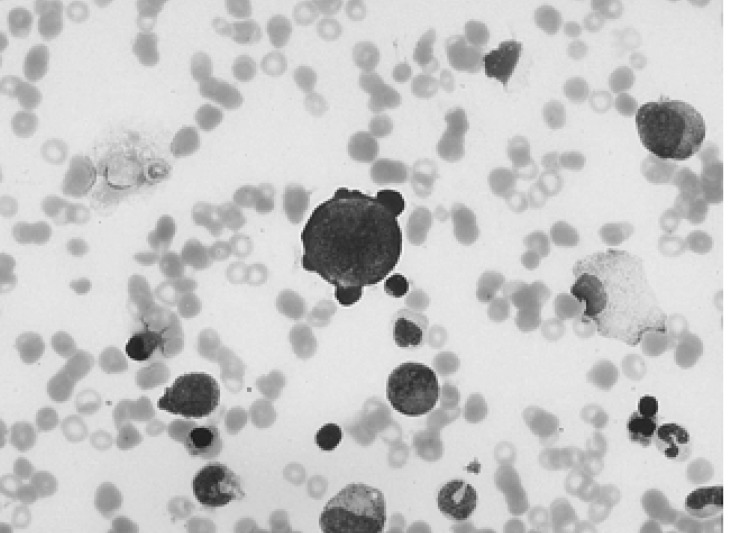
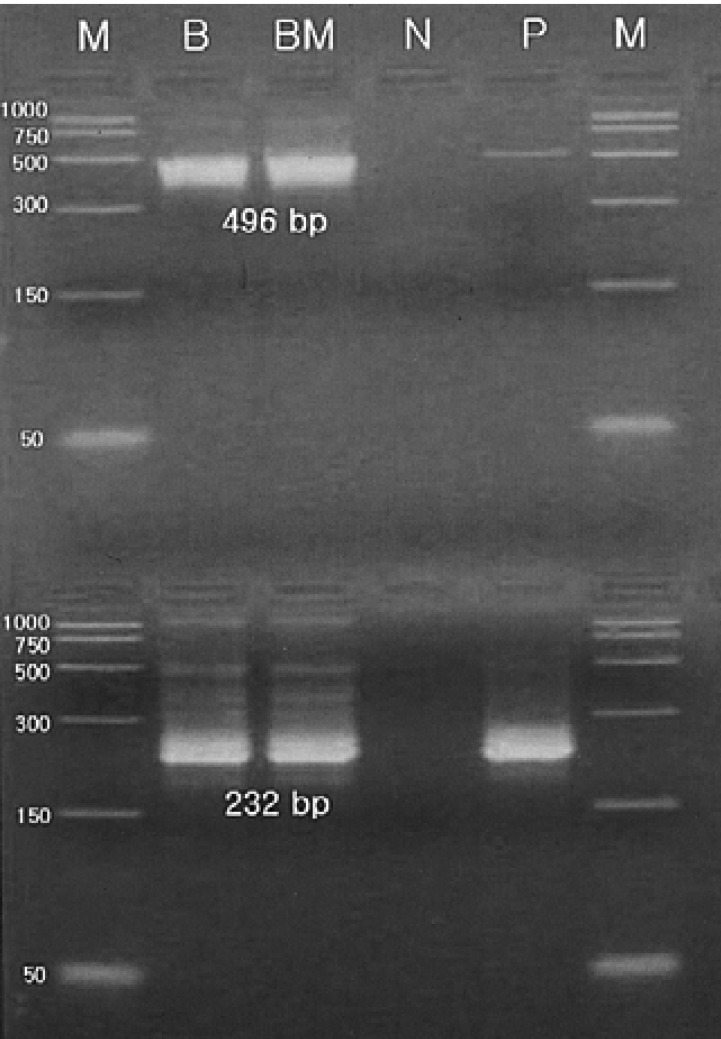
A 34-year-old female was admitted to the hospital with the chief complaint of presyncope, which had developed a few hours previously. She also complained of febrile sensation and myalgia which had begun 4 days prior to admission. She had no allergies or past medical history, and her family history was unremarkable. The physical examination revealed a blood pressure of 90/60 mmHg, a pulse rate of 96 beats per minute, a temperature of 37.8℃, and a respiration rate of 18 times per minute. Her skin and conjunctivae were pale, and sclera was yellowish. No evidence of cervical lymphadenopathy or abnormal findings upon chest examination was found. The low margin of the spleen was palpable at 5 cm from the costal margin. The initial complete blood counts were as follows: hemoglobin (Hb) 3.0 g/dL, hematocrit (Hct) 8.4%, MCV 81.7 fl, MCHC 36%, white blood cell (WBC) count 3,160/mm3 (segmented neutrophil 66%, lymphocytes 30.5%, monocytes 1.5%), platelets count 163,000/mm3, and reticulocytes 0.2%. Liver function tests showed an aspartate aminotransferase 81 IU/L, alanine aminotransferase 52 IU/L, total bilirubin 5.2 mg/dL with indirect bilirubin of 3.5 mg/dL, and lactate dehydrogenase 763 IU/L. Serum iron, total iron binding capacity, and ferritin level were within normal ranges, and haptoglobin had been decreased to 38.0 mg/dL. The peripheral blood smear revealed spherocytosis with reticulocytopenia and pancytopenia (Figure 1). The direct antiglobulin (Coombs) test was negative, and osmotic fragility of patient's red cells was increased. Bone marrow aspirate smears showed a few giant pronormoblasts with deep blue cytoplasm, pseudopods, and poorly demarcated intranuclear inclusions (Figure 2). These results indicate that the diagnostic hypothesis was an aplastic crisis induced by HPV B19 infection in a patient with underlying HS. Using the patient's peripheral blood and bone marrow aspirate, we performed polymerase chain reaction (PCR) for the detection of HPV B19 DNA. The PCR of the patient's materials resulted in an amplification of the 496 base pair (bp) band, which was confirmed by the nested PCR, where a 232 bp band was amplified (Figure 3). The patient was subsequently given eight units of packed red blood cells. During the course of admission, the WBC count decreased to 1,170/mm3 on the 2nd day and platelet count fell to 93,000/mm3 on the 4th day. We requested a serologic test for anti-HPV B19 IgM and IgG antibodies on the 10th day of hospitalization. Hematologic recovery began 12 days after admission. At discharge on the 15th day, the Hb was 8.4 g/dL, Hct 24.3%, WBC count 5,840/mm3, platelet count 233,000/mm3, and reticulocyte 5.1%. HPV B19 serology was pending at the time of discharge. One week later, the patient's Hb and Hct rose to 10.0 g/dL and 30%, with a reticulocyte count of 10%. Tests for anti-HPV B19 IgM and IgG antibodies were positive.
DISCUSSION
HPV B19 lytically infects erythroid progenitor cells and inhibits erythropoesis, leading to acute erythroblastopenia and reticulocytopenia10). When infected with the virus, healthy subjects may develop this to some degree, but the normal erythrocyte life span in these otherwise healthy subjects prevents them from becoming significantly anemic5). Although the major targets of HPV B19 are erythroid progenitor cells, HPV B19-induced bone marrow damage does not exclusively involve a single hematopoietic cell lineage. In addition to reticulocytopenia, neutropenia and thrombocytopenia have been observed in association with HPV B19 infection in patients both with and without hemolytic disorders7, 8, 11). Our patient also showed transient pancytopenia. Though not yet clearly explained, neutropenia and thrombocytopenia might be associated with hematophagocytosis or with HPV B19-induced inhibition of hematopoiesis7, 12).
The typical clinical picture of HPV B19 infection and subsequent aplastic crisis includes one or more of the following symptoms: fever, headache, myalgia, and abdominal pain. The length of time between infection and onset of aplastic crisis is known to be eight to ten days. The interval between the onset of symptoms and reappearance of reticulocytes in peripheral blood ranges from five to twenty days13). Fortunately, life-long immunity is generally acquired following infection with HPV B19, so most patients are subjected to no more than one such crisis in a lifetime14). However, such crises may be clinically severe and require prompt red cell transfusion. Our patient fits the classical clinical picture for aplastic crisis caused by HPV B19. After an unspecific presentation of febrile sensation and myalgia, she presented with presyncope which had probably been caused by profound anemia. She was subsequently transfused with red blood cell concentrates because of the severity of aplastic crisis. For patients with prolonged severe aplasia or immunodeficiency, intravenous immunoglobulin infusion for 5 to 10 days can often be recommended8). However, we chose not to administer immunoglobulin to our patient without immunodeficiency. Her hematologic recovery began at approximately 16 days after the onset of symptoms.
The earliest and most sensitive diagnostic method available is the identification of HPV B19 DNA using PCR in the bone marrow aspirates or blood sample8, 14, 15). Serologic tests for anti-HPV B19 IgM antibodies may be another useful diagnostic method14). Anti-HPV B19 IgM antibodies develop the second week after infection and may be detectable for ≤ 4~6 months. IgG antibodies appear several days after IgM, at the end of the second week or early in the third week. IgG antibodies remain in the system for the rest of the patient's life, generally signifying past infection. However, HPV B19 DNA amplification using blood samples may be difficult because high-titer viremia lasts only 2 to 3 days8). Serologic test is incapable of detecting acute infection at the onset of symptoms before the appearance of immunologic response14). Therefore, the contemporaneous determination of HPV B19 DNA by PCR and IgM antibody by serology seems to be the most appropriate diagnostic protocol in order to obtain correct laboratory diagnosis14). In this case, infection with HPV B19 was confirmed both by the detection of HBN DNA using PCR performed on the 3rd day of hospitalization, as well as the presence of anti-HPV IgM antibodies on a serologic test requested on the 10th day.
In the current paper, we described an aplastic crisis induced by HPV B19 in a patient with underlying HS. To the best of our knowledge, this is the first report in Korea that describes an adult with aplastic crisis as an initial presentation of HS.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





