 |
 |
| Korean J Intern Med > Volume 20(2); 2005 > Article |
|
Abstract
Background
Glucocorticoids have been known to be less effective for treating ankylosing spondylitis (AS) patients than for treating rheumatoid arthritis (RA) patients. To elucidate the mechanisms underlying this phenomenon, we evaluated whether the glucocorticoid receptor (GR)╬▓ expression of the peripheral blood mononuclear cells (PBMCs) in patients with AS is increased compared with patients with RA.
Methods
PBMCs were isolated from the subjects of 3 study groups: the healthy controls (n=25), the RA patients (n=25), and the AS patients (n=25). All the subjects had never taken corticosteroids and the patients with RA or AS were newly diagnosed. The expression of GR╬▓ messenger RNA (mRNA) was determined by reverse transcription of the total RNA, and this was followed by semi-quantitative polymerase chain reaction analysis (RT-PCR).
Results
The level of GR╬▒ mRNA expression was not different among three groups. GR╬▓ mRNA expression of the AS patients (2.02 [range: 0.99-7.21], median [25th-75th percentiles]) was enhanced compared with that of the controls (0.78 [range: 0.43-1.62]) and the RA patients (0.98 [range: 0.79-1.18]). The level of GR╬▓ mRNA expression was not related to the inflammatory markers or the disease activity score 28 for the RA patients, and it was not related to the Bath ankylosing spondylitis disease activity index for the AS patients.
Glucocorticoids were used therapeutically for the first time to treat a 28-year-old woman with crippling rheumatoid arthritis (RA)1), and the result was dramatic. Since then, glucocorticoids have earned a reputation as being the only drug that can reliably, effectively and rapidly suppresses the symptoms and signs of synovitis in RA. The evidence that glucocorticoids are effective, in the short term, on selected measures of disease activity has been reviewed in a recent meta-analysis2). However, to date, no studies have been performed to evaluate the effectiveness of continuous oral glucocorticoid treatment for ankylosing spondylitis (AS). According to the clinical experience, oral glucocorticoid treatment is clearly less effective for AS than for RA. The glucocorticoid receptor (GR) is essential for the action of glucocorticoid on various effector cells. The existence of two highly homologous glucocorticoid receptor isoforms, namely GR╬▒ and GR╬▓, which differ only at their C-termini, was predicted by sequencing the human GR cDNA and the gene3). Both GR╬▒ and GR╬▓ are products of alternative splicing of the primary transcript of GR messenger RNA (mRNA)3). GR╬▒ is a ligand-activated transcription factor that modulates the expression of glucocorticoid-responsive genes by binding to specific glucocorticoid response elements (GREs), whereas GR╬▓ does not bind glucocorticoids and it is transcriptionally inactive4). It has been suggested that GR╬▓ may be an endogenous inhibitor of glucocorticoid action and it may be an important dominant negative regulator for determining glucocorticoid sensitivity in the target tissues5). The increased expression of GR╬▓ was recently reported in patients with glucocorticoid-insensitive asthma and ulcerative colitis6,7). To test the possibility that the ineffectiveness of oral glucocorticoid for the treatment of AS may be associated with a higher expression of GR╬▓, we compared the expression of GR╬▓ in newly diagnosed RA and AS patients.
Twenty five RA patients and 25 AS patients were recruited from the outpatient clinic of Asan Medical Center in Korea from January 2001 to November 2001. The diagnosis of AS was made when the patients met the modified New York criteria8). A diagnosis of RA was made when the patients fulfilled the American Rheumatism Association 1987 revised criteria for the classification of RA9). Twenty five healthy volunteers were recruited as controls. The patients with AS or RA were newly diagnosed and they had never taken glucocorticoids. Informed consents were obtained from all the patients and controls. Heparinized blood (30 mL) was donated by the patients with AS or RA, and the healthy controls. Peripheral blood mononuclear cells (PBMCs) were isolated from these blood samples by the Ficoll-paque density gradient method (Amersham Pharmacia Biotech, Uppsala, Sweden).
The demographic data of the patients were recorded. The acute phase reactants, the erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), were measured. The ESR was calculated by the Westergren method (normal 0-9 mm/hr) and the CRP was measured quantitatively by immunonephelometry (N Latex CRP mono, Behring Diagnostics, San Jose, CA, USA) (normal CPR level: 0-0.6 mg/dL). In the current study, the disease activity of AS is reported in the form of Bath Ankylosing Spondylitis Disease Activity Index (BASDAI)10). The disease activity of RA is reported in the form of 28 Disease Activity Score (DAS28), which is a validated composite index measuring the swollen joint count (28 joints), the tender joint count (28 joints), the ESR and the patient's overall assessment of well-being11).
The expression of GR╬▒ and GR╬▓ mRNA was determined by reverse transcription of the total RNA followed by polymerase chain reaction analysis (RT-PCR). The RNA was extracted by using an RNA Isolation Kit (Promega, Madison, WI, USA). The cDNAs were synthesized by extension of the oligodT primers (Promega) with using mouse mammary leukemia virus (MMLV) reverse transcriptase (Promega). PCR of the cDNAs was performed with the common upstream primer (5'-CCTAAGGAC GGTCTGAAGAGC-3', corresponding to nucleotides 2158-2178 of the GR╬▒ and GR╬▓ cDNA) and the specific downstream primers (GR╬▒: 5'-GCCAAGTCTTGGCCCTCTAT-3', complementary to nucleotides 2616-2635 of GR╬▒ cDNA; and GR╬▓:5'-CCACGTATC CTAAAAGGGCAC-3', complementary to nucleotides 2503-2523 of GR╬▓ cDNA). The transcript of human glucose-3-phosphate dehydrogenase (GPDH) was amplified from the same cDNA samples to serve as a control for sample loading and integrity. The PCR of GPDH was performed with a sense primer (5'-ACCACAGTCCATGCCATCAC-3') and an antisense primer (5'-CCACCACCCTGTTGCTGCA-3'). Each PCR procedure was carried out in a total volume of 50 ┬ĄL containing 34.75 ┬ĄL of distilled water, 5 ┬ĄL of cDNA, 5 ┬ĄL of 10 x PCR buffer (Promega) 1 ┬ĄL of each primer, 0.25 ┬ĄL of Taq DNA polymerase, 1 ┬ĄL of dNTP and 3 ┬ĄL of MgCl2. After an initial denaturation period of 5 minutes at 95Ōäā, and then 36 cycles (for GR╬▒ and GPDH) or 46 cycles (for GR╬▓), consisting of incubations at 94Ōäā for 30 seconds, 60Ōäā for 30 seconds, and 72Ōäā for 1 minute, were carried out. After a final extension step for 10 minutes at 72Ōäā, the PCR products were separated by electrophoresis on a 1.0% agarose gel and then they were visualized by ethidium bromide staining. The intensity of the ethidium bromide fluorescence was measured densitometrically. The density of each PCR products was adjusted according to the density of GPDH.
Statistical differences in the expressions of GR╬▒ and GR╬▓ mRNA were tested for by the Kruskal-Wallis test and the Mann-Whitney test. The relationships between the expressions of GR and GR mRNA and the ESR, CRP, BASDAI, DAS28 and RF were analyzed by the Spearman correlation test. p values of <0.05 were considered as being statistically significant.
The demographic, clinical and laboratory data are shown in Table 1. As expected, their were differences for age and gender between the RA and AS patients. The elevated values of the mean ESR and the mean CRP were observed in both the RA and AS patients. For those patients with RA, 22 patients (80%) were rheumatoid factor positive. The mean DAS 28 was 4.3┬▒0.9 (mean┬▒standard deviation), with a range of 2.3-6.9, which indicated the active stage of RA. In patients with AS, 25 patients (100%) were positive for human leukocyte antigens (HLA) B27. The mean BASDAI was 3.7┬▒2.1, with a range of 1.3-9.8.
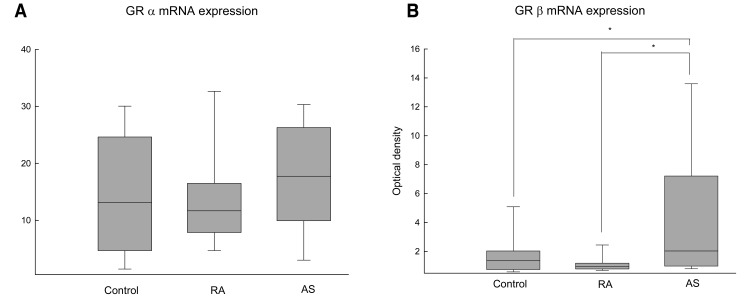
As shown in Figure 1A, the RT-PCR product of GR╬▒ mRNA (477 base pairs [bp]) was detectable in all the samples we tested including the healthy controls and the patients with RA or AS. The level of expression of GR╬▒ mRNA, when normalized to GPDH, were not different among the three groups (control: 13.09 [range: 4.70-24.63] {median [25th-75th percentiles]}, RA: 11.64 [range: 7.87-16.49], AS: 17.74 [range: 9.92-26.26], p=0.34) (Figure 2A). Among the 25 patients with AS, the RT-PCR product of GR╬▓ mRNA (365 bp) was detected in 17 patients (68%). In contrast, GR╬▓ mRNA was detected in only 5 of the 25 patients with RA (20%) and in only 9 of the 25 healthy controls (30%) (Figure 1B). The expression level of GR╬▓ mRNA, when normalized to GPDH, was higher in patients with AS (2.02 [range: 0.99-7.21]) than that of the RA patients (0.98 [range: 0.79-1.18]) and the controls (0.78 [range: 0.43-1.62]) (p=0.01) (Figure 2B).
To assess what clinical factors are associated with the expression of GR╬▒ and GR╬▓ mRNA, BASDAI, DAS28, ESR, CRP, RF, the tender joint count, the swollen joint count, the patients' global assessment (PGA), and their visual analogue pain scale (VAS, 0-10 cm) were analyzed by Spearman correlation testing. For the patients with RA, the ESR, CRP, RF, the tender joint count, the swollen joint count, PGA, VAS and DAS 28 were not correlated with the expression of GR and GR mRNA. For the patients with AS, the ESR, CRP, and BASDAI were not correlated with the expression of GR╬▒ and GR╬▓ mRNA.
Glucocorticoids are used widely as treatment for the suppression of inflammation in chronic inflammatory diseases. However, some of the patients with autoimmune diseases who are treated with glucocorticoids often fail to respond to glucocorticoids. This is called immune system-specific glucocorticoid resistance and it appears to be the results of tissue-specific defects of the GR transduction system12,13). This condition might be genetically/constitutionally determined. There might be several mechanisms responsible for this phenomenon, including changes in the intracellular hormone availability, the levels of cellular GR╬▒ and the hormone-binding affinity. Other mechanisms could involve phosphorylation, nuclear translocation, GRE binding and/or its interaction with other nuclear factors, as well as abnormally high levels of GR╬▓14-16).
Based on clinical experience, glucocorticoid treatment is known to be less effective for AS than RA17). Among the several mechanisms of glucocoriticoid resistance, we investigated the levels of GR╬▓ in the PBMCs from patients with RA or AS. In the present study, we demonstrated that GR╬▓ mRNA expression was significantly increased in the PBMCs from patients with AS compared with that of the healthy controls and the patients with RA. The pathophysiological factors that induce the enhanced expression of GR╬▓ mRNA in AS patients are not yet clear. It may be possible that systemic inflammation may cause the increase expression of GR╬▓ mRNA in AS patients. However, we did not find any correlation between the inflammatory markers (ESR and CRP) and the expression level of GR╬▓ mRNA in the AS patients. BASDAI also did not affect the level of expression of GR╬▓ mRNA in the AS patients. For the patients with RA, the DAS 28, which included the swollen joint count, the tender joint count, ESR and the patients' overall assessment of well-being, was not associated with the expression of GR╬▓ mRNA. These results suggest that GR╬▓ mRNA expression in the PBMCs is not induced by an inflammatory reaction for the patients with AS or RA.
A genetically determined imbalance of the glucocorticoid receptor isoforms was found in the cultured lymphocytes from a patient suffering from chronic leukemia and having congenital generalized glucocorticoid resistance18). This patient presented with a greatly reduced GR╬▒ level and a normal GR╬▓ level, and this resulted in a low GR╬▒ to GR╬▓ ratio. However, there was no difference for the GR╬▒ mRNA expression among the three groups in our study. For the patients with AS, the decreased expression of GR╬▒ mRNA was not found compared to the patients with RA.
It is unclear if a low dose of glucocorticoid can induce GR expression7,19). Korn et al have reported an unchanged splicing level of GR╬▓ mRNA in bronchial epithelial cells after they were exposed to glucocorticoid reagents in vivo19). To exclude the potential effect of glucocorticoids on the splicing of GR╬▓ mRNA, we selected patients who have never taken glucocorticoid.
The various effects of glucocorticoid are mediated by specific intracellular glucocorticoid receptors. GR╬▒ and GR╬▓ are products of alternative splicing in exon 9 of the gene encoding GR, and this gene is located on chromosome 53). In contrast to GR╬▒, GR╬▓ does not bind glucocorticoids in vitro and it is transcriptionally inactive on a GRE-containing enhancer20). The expression of GR╬▓ mRNA has been demonstrated by RT-PCR for a variety of human tissues, but the level of GR╬▓ mRNA was relatively low and it was considered to be 0.2~0.3% of the GR╬▒ mRNA14). Bomberger et al. showed that the increased production of GR╬▓ compared with GR╬▒ could disrupt the enhancing effects of GR╬▒ in COS-7 cells4). Oakely et al. also demonstrated the GR╬▓ dominant negative activity in HeLa-S3 cells that have endogenous GR╬▒ receptors, and this activity was also regulated in a GRE-mediated manner20). The potential mechanisms responsible for this dominant negative effect exerted by GR╬▓ have not yet been elucidated. The mechanism might involve competition between GR╬▒ and GR╬▓ for GRE binding, formation of transcriptionally inactive or partially active GR╬▒-GR╬▓, and/or competition for the stoichiometrically limited concentrations of the coactivators that are needed by GR╬▒ for full transcriptional activity 21, 22).
On the contrary, Hecht et al. found no evidence for a specific dominant negative effect of GR╬▓ on the trans-activation induced by GR╬▒23). Also, cotransfection of GR╬▓ in primary human lymphocytes or Jurkat T lymphoma cells had no effect on trans-activation by the GR╬▒ isoforms, even when the GR╬▓ protein was found at greater than five times the levels of GR╬▒24).
These discrepancies seem to be the results of using different vectors, the different cell/tissue specificities and the limitations of the cotransfection experiments. In contrast to the controversial nature of in vitro experiments at the cellular level of GR╬▓ action, evidence is now accumulating that indicates the relationship between the GR╬▓ expression and the glucocorticoid responsiveness of organs or individuals in vivo. Hamid et al. reported that the GR╬▓ postitive PBMCs or bronchoalveolar lavage cells are significantly increased in glucocorticoid-resistant patients with bronchial asthma6). Honda et al. showed that the positive rate of GR╬▓ mRNA detected by RT-PCR was higher for glucocorticoid resistant ulcerative colitis patients than for glucocorticoid sensitive patients7). Our results also showed the relationship between glucocorticoid unresponsiveness and GR╬▓ mRNA expression in AS patients. Further experiments regarding glucocorticoid binding affinity, the rate of GR nuclear translocation and it interaction with other nuclear factor are required to reveal the mechanisms that are responsible for the ineffectiveness of glucocorticoids in AS patients.
In summary, we found the increased expression of GR╬▓ mRNA in AS patients compared with RA patients. Theses previously unreported results suggest the possibility that the increased expression of GR╬▓ may be associated with the ineffectiveness of continuous oral glucocorticoid treatment for AS.
ACKNOWLEDGEMENT
This study was supported by a grant (grant number 2001-066) from the Asan Institute for Life Sciences, Seoul, Korea.
References
1. Hench PS, Kendall EC, Slocumb CH, Polley HF. Effects of a hormone of the adrenal cortex (17-hydroxy-11dehydrocorticosterone: compound E) and of pituitary adrenocorticotropic hormone on rheumatoid arthritis. Mayo Clin Proc 1949;24:181ŌĆō197.
2. Gotzsche PC, Johansen HK. Meta-analysis of short term low dose prednisolone versus placebo and non-steroidal anti-inflammatory drugs in rheumatoid arthritis. BMJ 1998;316:811ŌĆō818PMID : 9549450.



3. Hollenberg SM, Weinberger C, Ong ES, Cerelli G, Oro A, Lebo R, Thompson EB, Rosenfeld MG, Evans RM. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature 1985;318:635ŌĆō641PMID : 2867473.



4. Bamberger CM, Bamberger AM, de Castro M, Chrousos GP. Glucocorticoid receptor beta, a potential endogenous inhibitor of glucocorticoid action in humans. J Clin Invest 1995;95:2435ŌĆō2441PMID : 7769088.



5. Bamberger CM, Shulte HM, Chrousos GP. Molecular determinants of glucocorticoid receptor function and tissue sensitivity to glucocorticoids. Endocr Rev 1996;17:245ŌĆō261PMID : 8771358.


6. Hamid QA, Wenzel SE, Hauk PJ, Tsicopoulos A, Wallaert B, Lafitte JJ, Chrousos GP, Szefler SJ, Leung DY. Increased glucocorticoid receptor beta in airway cells of glucocorticoid-insensitive asthma. Am J Respir Crit Care Med 1999;159:1600ŌĆō1604PMID : 10228133.


7. Honda M, Orii F, Ayabe T, Imai S, Ashida T, Obara T, Kohgo Y. Expression of glucocorticoid receptor beta in lymphocytes of patients with glucocorticoid-resistant ulcerative colitis. Gastroenterology 2000;118:859ŌĆō866PMID : 10784585.


8. van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis: a proposal for modification of the New York critieria. Arthritis Rheum 1984;27:361ŌĆō368PMID : 6231933.


9. Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, Medsger TA, Mitchell DM, Neustadt DH, Pinals RS, Schaller JG, Sharp JT, Wilder RL, Hunder GG. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315ŌĆō324PMID : 3358796.


10. Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis. J Rheumatol 1994;21:2286ŌĆō2291PMID : 7699630.

11. Prevoo ML, van't Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts: development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44ŌĆō48PMID : 7818570.


13. Szefler SJ, Leung DY. Glucocorticoid resistant asthma: pathogenesis
and clinical implications for management. Eur Respir J 1997;10:1640ŌĆō1647PMID : 9230260.


14. Reichardt HM, Kaestner KH, Tuckerman J, Kretz O, Wessely O, Bock R, Gass P, Schmid W, Herrlich P, Angerl P, Schutz G. DNA binding of the glucocorticoid receptor is not essential for survival. Cell 1998;93:531ŌĆō541PMID : 9604929.



15. Scheinman RI, Gualberto A, Jewell CM, Cidlowski JA, Baldwin AS Jr. Characterization of mechanisms involved in transrepression of NF-kappa B by activated glucocorticoid receptors. Mol Cell Biol 1995;15:943ŌĆō953PMID : 7823959.



16. Leung DY, Hamid Q, Vottero A, Szefler SJ, Surs WJ, Minshall E, Chrousos GP, Klemm DJ. Assoication of glucocorticoid insensitivity with increased rexpression of glucocorticoid receptor beta. J Exp Med 1997;186:1567ŌĆō1574PMID : 9348314.



17. Dougados M, Dijkmans B, Khan M, Maksymowych W, van der Linden S, Brandt J. Conventional treatments for ankylosing spondylitis. Ann Rheum Dis 2002;61(Suppl 3):iii40ŌĆōiii50PMID : 12381510.



18. Shahidi H, Vottero A, Stratakis CA, Taymans SE, Karl M, Longui CA, Chrousos GP, Daughaday WH, Gregory SA, Plate JM. Imbalanced expression of the glucocorticoid receptor isoforms in cultured lymphocytes from a patient with systemic glucocorticoid resistance and chronic lymphocytic leukemia. Biochem Biophys Res Commun 1999;254:559ŌĆō565PMID : 9920778.


19. Korn SH, Wouters EF, Wesseling G, Arends JW, Thunnissen FB. In vitro and in vivo modulation of alpha- and beta-glucocorticoid-receptor mRNA in human bronchial epithelium. Am J Respir Crit Care Med 1997;155:1117ŌĆō1122PMID : 9116996.


20. Oakley RH, Sar M, Cidlowski JA. The human glucocorticoid receptor beta isoform: expression, biochemical properties, and putative function. J Biol Chem 1996;271:9550ŌĆō9559PMID : 8621628.


21. Sheppard KA, Phelps KM, Williams AJ, Thanos D, Glass CK, Rosenfeld MG, Gerritsen ME, Collins T. Nuclear integration of glucocorticoid receptor and nuclear factor-kappa B signaling by CREB-binding protein and steroid receptor coactivator-1. J Biol Chem 1998;273:29291ŌĆō29294PMID : 9792627.


22. de Lange P, Koper JW, Huizenga NA, Brinkmann AO, de Jong FH, Karl M, Chrousos GP, Lamberts SW. Differential hormone dependent transcriptional activation and repression by naturally occurring human glucocorticoid receptor variants. Mol Endocrinol 1997;11:1156ŌĆō1164PMID : 9212062.


Figure┬Ā1
Expression of GR mRNA by RT-PCR. (A) Products of GR╬▒ mRNA (477 bp) were detected in all samples. (B) Products of GR╬▓ mRNA (365 bp) were detected in 30% of the control samples, in 20% of the RA samples and in 68% of the AS samples.

Figure┬Ā2
Densitometric analysis of GR mRNA expression normalized to GPDH. (A) The level of GR╬▒ mRNA expression was not different among three groups. (B) The expression of GR╬▓ mRNA was higher for AS patients than that for the RA and control patientss. Boxes represent the 25th to 75th percentile; vertical lines indicate the 5th and 95th percentile. Statistical difference is indicated as *p < 0.05 by Mann-Whitney test.
Table┬Ā1
Demographic, clinical and laboratory data
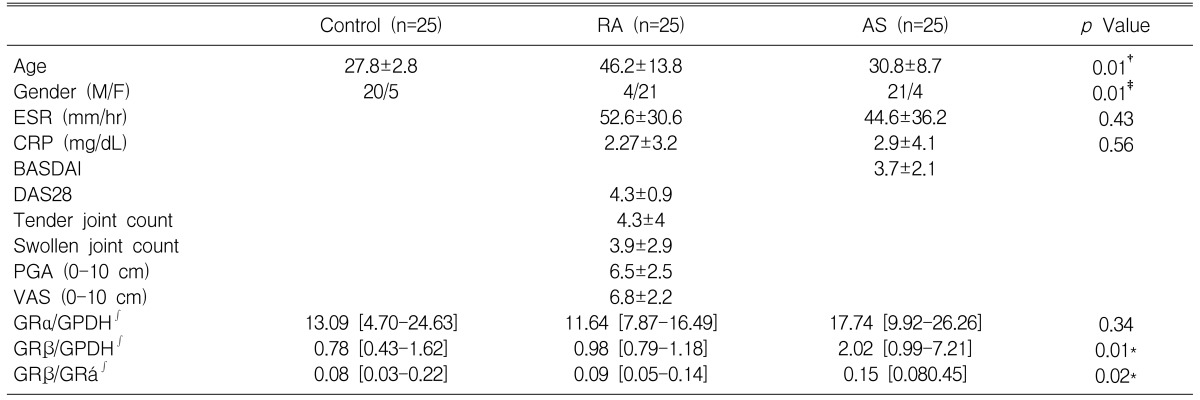
Values are means ┬▒ standard deviation. Ōł½ is expressed as the median [25th-75th percentiles].
RA, rheumatoid arthritis; AS, ankylosing spondylitis; M, male; F, female; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; DAS28, Disease Activity Score 28; PGA, patient global assessment; VAS, visual analogue pain scale; GR╬▒, glucocorticoid receptor alpha; GR╬▓, glucocorticoid receptor beta
ŌĆĀp<0.05 by ANOVA, ŌĆĪp<0.05 by chi-square test, *p<0.05 by Kruskal-Wallis test
-
METRICS

- Related articles
-
The usefulness of trabecular bone score in patients with ankylosing spondylitis2021 September;36(5)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


