 |
 |
| Korean J Intern Med > Volume 18(4); 2003 > Article |
|
Abstract
Background:
Since its reemergence in 1993, a number of cases of Plasmodium vivax malaria have been reported in Korea. We analyzed the cases of malaria patients living in Chuncheon and its neighboring communities, to characterize its clinical manifestations and laboratory findings, and to identify any differences between our clinical findings and those of previous studies.
Methods:
We reviewed the clinical records of cases that were confirmed as malaria by peripheral blood smear at Chuncheon Sacred Heart Hospital from July 1998 to September 2001.
Results:
Forty-four cases were included in the study. All patients were infected with Plasmodium vivax, and presented with high fever; however, tertian fever developed in only 15 patients (35.7%). A number of cases showed various symptoms, which included headache, abdominal pain, nausea and vomiting. Of the 44 cases identified, 41 (93.2%) developed malaria between June and September.
Thrombocytopenia was a prominent finding in 75% of the cases at diagnosis, but resolved during or after therapy. Other laboratory abnormalities such as, anemia, elevated transamines, coagulopathies, and elevated lactose dehydrogenase (LDH) were also noted. Cerebrospinal fluid (CSF), studies were performed in five cases, one of which showed pleocytosis in the CSF.
Conclusion:
We noted only 15 patients (35.7%) with tertian fever; the other patients showed variable fever patterns. Thrombocytopenia was the most prominent laboratory finding. Therefore, we suggest that malaria should be included in the differential diagnosis of febrile diseases with an onset between June to and September, regardless of the pattern of the fever.
Plasmodium vivax malaria has re-emerged as an endemic disease in Korea, after an extended period of effective control. The incidence of malaria on a global scale has decreased recently as a direct result of on-going anti-malaria campaigns conducted by governments and the World Health Organization (WHO) since the 1960s. In 1984, only two cases of malaria were reported in Korea, and the disease seemed to have been eradicated in Korea1). A few cases of imported malaria were reported during the following ten years. However, in 1993 a case of vivax malaria, was reported in an individual who had never been abroad2). Subsequently, the annual incidence of vivax malaria increased in both the military and civilians populations near the Demilitarized Zone (DMZ), and this continued until 2000. However, the number of cases of malaria has decreased since 2000 because of a reduction in the number of Anopheles mosquitoes, due to drought conditions and to the intensified malaria control activities undertaken in 2001. The decrease in the number of cases among soldiers was greater than the decrease observed in the civilian population; such decreases were more prominent in Paju-Gun, Yeoncheon-Gun and Cheorwon-Gun than they were elsewhere. Nevertheless, 2,522 cases of malaria were diagnosed in 2001, and therefore, a comprehensive malaria control and treatment program is needed1).
Several studies of vivax malaria infection in Korea have been undertaken, but most of the subjects in these studies were soldiers or veterans, and most had tertian fever3–5). We studied the characteristics of civilian cases (including veterans) with vivax malaria living either in or near Chuncheon. We compared the results of our study with those of previous studies, and we hope that our results will be helpful in the diagnosis and treatment of vivax malaria.
We retrospectively studied the medical records of 44 cases diagnosed with malaria by peripheral blood smears between July 1998 and September 2001 at Chuncheon Sacred Heart Hospital. We analyzed the clinical and laboratory features of malaria, including the fever pattern, place of residence, onset time, complete blood counts and coagulation times, and liver function. Patients were treated with hydroxychloroquine, followed by primaquine for 2 weeks. The laboratory findings from pre- and post-treatment testing were compared using paired t-tests for median values. On average, follow-up tests were performed 8.7 days after pre-treatment testing.
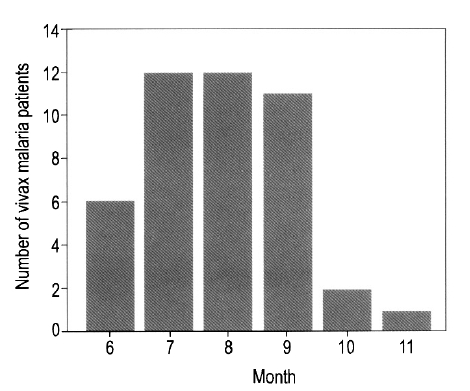
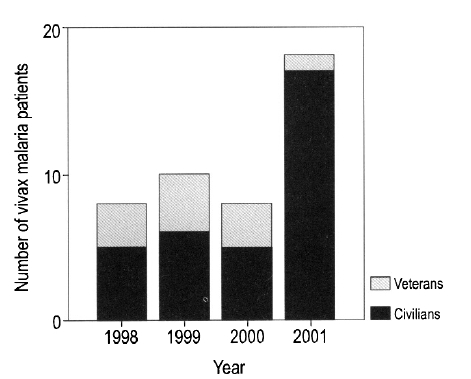
Forty-four cases were diagnosed with vivax malaria; no cases of falcifarum malaria were identified. Of the 44 cases, 33 were male (75%), and the mean age of the 44 cases was 35±16.7 years. Forty-one cases (93.2%) presented between the months of June and September, two cases presented in October, and one case in November (Figure 1). Eight cases were diagnosed in 1998, 10 in 1999, 8 in 2000, and 18 in 2001. The proportion of veterans among the affected cases ranged from 37.5% to 40% between 1998 and 2000; however, only one veteran (5.6%) was diagnosed in 2001 (Figure 2). Among the veterans (n=11), two patients had histories of previous malaria infection and treatment. On average, 7.7±6.8 days passed between the onset of symptoms and presentation at the hospital. Most cases received symptomatic treatments.
Of the 44 cases, 26 (59.1%) lived in Chuncheon. The other regions were: Yang Gu Gun, Gapyeong Gun, Hwacheon Gun, Inje Gun, and Hongcheon Gun. In 2001, the proportion of patients living in Chuncheon decreased to 33.3%.
Fever and chills developed in all cases. Of the forty-two cases whose fever patterns were noted in the records, 15 (35.7%) developed tertian fever; 22 (54.8%) had fevers daily, and 5 had irregular fever patterns (Figure 3). Thirteen cases (29.5%) complained of abdominal pain, and 15 cases (34.1%) had gastrointestinal symptoms such as nausea, vomiting, and diarrhea.
Pre-treatment, the mean leukocyte count was 5,155±1,562 cells/mm3, mean hemoglobin 12±2.1 g/dL, and mean platelet count 106,000±82,000 platelets/mm3. Thirty-three cases (75%) developed thrombocytopenia. In nine cases (20.5%), the platelet count was less than 50,000/mm3 (Table 2). None of the cases showed signs of bleeding, despite the presence of severe thrombocytopenia.
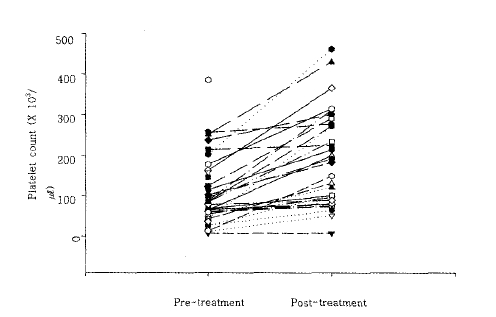
We performed follow-up laboratory testing during treatment or post-treatment in 30 cases. At follow-up, the mean leukocyte count was 6,036±1,955 cells/mm3, and the mean platelet count 192,200±118,000 platelets/mm3. Both counts were significantly higher than those obtained at the time of diagnosis. In 11 cases who did not develop thrombocytopenia, platelet counts increased significantly from pre- to post-treatment, from 215,000±36,295/mm3 to 339,4288±4,654/mm3. The increase in platelet counts observed during treatment was a pronounced finding (Figure 4).
Other laboratory abnormalities were noted, including elevated LDH in 87.5% of cases, as well as a prolonged prothrombin time (PT) and a prolonged activated partial prothrombin time (aPTT), in 71.9% and 78.2% of cases, respectively (Table 2).
Cerebrospinal fluid analysis was performed on 5 of 28 cases with headaches; in one of the five CSF samples, the leukocyte count was 63 cells/mm3. Twenty-three cases (52.3%) showed splenomegaly by physical examination, or abdominal ultrasonography.
After the re-emergence of vivax malaria in 1993, its incidence steadily increased until 2000, but then decreased in 20011). At the beginning of this endemic period, the majority of cases were soldiers based near the DMZ area, but since 1999 more civilian than military cases have been reported1). In 2001, there were 1,113 civilian cases, 754 veteran cases and 685 among military personnel. The proportion of cases accounted for by veterans has decreased from between 37.5 to 40% in previous years to only 5.6% in 2001. These findings indicate that the control programs used by the military have been effective, and that civilians should be similarly educated about the avoidance and control of malaria.
Vivax malaria is transmitted by the bite of an infected Anopheles mosquito, and infection shows characteristic symptoms such as, tertian fever and severe chills. In addition, rupture of red cell by schizonts stimulates the release of several inflammatory cytokines, which cause fever and myalgia. At first, the fever is irregular but, with time, nearly synchronous red cell rupture causes the typical cyclic fever6). In several studies of indigenous malaria in Korea, most cases of malaria have shown the classic cyclic fever pattern3–5). However, we noted that only 37.5% of all cases showed classic tertian fever, which represent a divergence from most Korean reports; however, Stanley found that only one third of travelers with malaria suffered from tertian fever6), and Kim. et al. reported that only 40% of 17 cases with indigenous malaria showed tertian fever in Korea7). These results are similar to ours.
The diagnoses of malaria were made early after the onset of fever in many cases; in nine cases, daily fever was noted 7 days after the onset of fever. In five cases, who were diagnosed 2 weeks or more after the onset of fever, daily fever was noted, or the pattern of fever was not determined. Eleven of 15 cases who showed tertian fever were diagnosed within one week of the onset of fever. Our study shows that the pattern of fever in vivax malaria cases varies considerably.
Seventy-five percent of all malaria cases had thrombocytopenia; this is similar to the results of many other studies2,5–9). Immunoglobulins directed against platelets destroy platelets in the spleen10), and malaria parasites can also destroy platelets directly. It has been suggested that the activation of the coagulation cascade may also reduce platelet counts11). Lim et al. suggested that platelet counts in malaria cases could be used as a simple and useful marker for post-treatment follow-up8).
Our study produced similar evidence concerning the utility of platelet count as a marker of the presence and progress of malaria. Both thrombocytopenic malaria cases and cases with normal platelet counts showed significant increases in platelet counts during, or after, treatment. Since over 90% of all malaria cases develop symptoms in the period from June to September, and since many cases had thrombocytopenia, we suggest that thrombocytopenia may be a critical clue for the diagnosis of malaria in febrile cases during those months.
Anemia in cases with malaria occurs as a result of the lysis of red cells by schizonts, bone marrow suppression, and splenic sequestration7). Our study also revealed that about 50% of all cases had anemia, which is less than the proportion reported by Kim et al7), but comparable to that reported by Oh et al5). In the our study, the hemoglobin level was found to increase after treatment, but not significantly. In cases where the hemoglobin level was severely depressed at diagnosis, the level returned to the normal range after therapy. Pre-treatment leukocyte counts were in the normal range, or slightly reduced, and increased significantly with treatment.
Malaria infection activates the coagulation cascade and induces consumptive coagulopathy. Hwang et al. reported that most patients with malaria showed prolonged aPTT, increases of D-dimer, and that one in twenty cases showed prolonged PT12). However, in the current study, the majority of patients showed both prolonged PT and prolonged aPTT. Three of these patients were tested for D-dimer, and all three showed increased D-dimer levels.
It is generally understood that vivax malaria does not induce meningitis, despite the presence of a severe headache13). In the present study, one case was found to have elevated leukocyte. counts in the CSF. Falciparum malaria may cause cerebral malaria due to cerebral sequestration of infected red blood cells, but this has not been found in vivax malaria. Jakobsen et al. suggested that vascular inflammation may occur, regardless of cerebral sequestration, because of increases in the levels of vascular adhesion molecules such as, sICAM, in vivax malaria-infected serum14). We believe that additional studies on meningitis related to vivax malaria are warranted.
In this study, many cases with vivax malaria did not show a cyclic tertian fever pattern, and in this respect our study differs from previous studies. Our laboratory findings showed that thrombocytopenia was particularly marked, and that the platelet count increased significantly after treatment. Many cases also developed coagulopathies. We advise that malaria should be included in the differential diagnosis of febrile diseases that develop from June to September, regardless of the fever patterns observed.
Table 1.
Clinical manifestations of cases with vivax malaria
| No. of patients (n=44) | Percent (%) | |
|---|---|---|
| Fever | 44 | 100 |
| Tertian fever | 15/42 | 35.7 |
| Headache | 28 | 63.6 |
| Abdominal pain | 13 | 29.5 |
| GI symptoms* | 15 | 27.8 |
Table 2.
Laboratory findings in cases with vivax malaria
REFERENCES
1. Ministry of Health and Welfare. 2002 Management Guidelines for Malaria. Ministry of Health and Welfare, 2002.
2. Chai IH, Lim GK, Yoon SN, Oh WI, Kim SJ, Chai JY. Occurrence of tertian malaria in a male patient who has never been abroad. Korean J Parasit 32:1995–20001994.

3. Lim CS, Kim YK, Lee KN, Kim KH, Kim HS, Lim HW, Kim BS, Kim JS. Hematologic findings of reappeared Plasmodium vivax in Korea. Korean J Clin Pathol 16:836–8431996.
4. Kim KH, Lim CS. Clinical observations in 26 cases of indigenous malaria in 1995. Korean J Med 52:577–5831995.
5. Oh MD, Shin H, Shin D, Kim U, Lee S, Kim N, Choi MH, Chai JY, Choe KW. Clinical features of vivax malaria. Am J Trop Med. Hyg 65:143–1462001.


7. Kim EO, Lee MS, Lee SO, Lee SH, Kim YS, Woo JH, Chi HS, Ryu JS. Clinical characteristics of malaria in Korean patients. Korean J Infect Dis 30:410–4151998.
8. Lim CS, Kim YK, Lee KN. Clinical significance of platelet index in posttreatment follow-up of vivax malaria. Korean J Infect Dis 30:407–4101998.
9. Kakar A, Bhoi S, Prakash V, Kakar S. Profound thrombocytopenia in Plasmodium vivax malaria. Diagn Microbiol Infect Dis 35:243–2441999.


10. Yamaguchi S, Kubota T, Yamagishi T, Okamoto K, Izumi T, Takada M, Kanou S, Suzuki M, Tsuchiya J, Naruse T. Severe thrombocytopenia suggesting immunological mechanisms in two cases of vivax malaria. Am J Hematol 56:183–1861997.


11. Srichaikul T. Hemostatic alterations in malaria. Southeast Asian I Trop Med Public Health 24:86–911993.
12. Hwnag SH, Chi HS, Woo JH, Lew JS. Hemostatic alteration in malaria infection. Korean J Hemost and Thromb 7:17–242000.
13. In: Brounwald E, Fauci AS, Kasper DL, Hauser SL, Longo DL, Jameson JL, eds. Harrison”s Principles of Internal Medicine. 15th ed. 1205. McGraw-Hill, USA: 2001.
-
METRICS

- Related articles
-
Clinical features of snoring patients during sedative endoscopy2019 March;34(2)
Clinical features of elderly chronic urticaria2014 November;29(6)
Clinical Features of Crohn`s Disease in Korea1987 July;2(2)
Clinical Features of Interstitial Lung Diseases1996 July;11(2)
Clinical Features of Ulcerative Colitis in Korea1996 January;11(1)




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


