 |
 |
| Korean J Intern Med > Volume 20(3); 2005 > Article |
|
Abstract
Background
Glutathion S-transferase P1 (GSTP1), the abundant isoform of glutathione S-transferase in lung epithelium, plays an important role in cellular protection against oxidative stress and toxic foreign chemicals. GSTP1 (Ile105Val) polymorphism has been reported to be associated with asthma related phenotypes such as atopy and bronchial hyperresponsiveness. Therefore we investigated whether this polymorphism may be associated with the development of aspirin-intolerant asthma (AIA).
Methods
GSTP1 Ile105Val polymorphism was determined using a single based extension method in 88 AIA subjects and compared to 154 aspirin-tolerant asthma (ATA) subjects and 119 normal healthy controls (NC) recruited from the Korean population.
Aspirin-intolerant asthma (AIA) is characterized by the development of asthma after taking aspirin and non-steroidal anti-inflammatory drugs (NSAIDs)1). The pathogenesis of AIA remains unclear, but many investigators have suggested that AIA is likely to be related with abnormal eicosanoid metabolism, in particular overproduction of leukotrienes (LTs)2-5).
Inflammation of the airways is a characteristic feature of asthma. Oxidative stress, including reactive oxygen species (ROS), may be one of the key components of airway inflammation resulting from the production of cytotoxic products such as lipid hydroperoxides and hydroxyradicals6). These products are substrates of GSTP1, which are essential in the mobilization of arachidonic acid and regulation of proinflammatory eicosanoid release such as LTs and prostaglandins7). Inability to detoxify ROS can perpetuate the inflammation process, activate bronchoconstriction response and finally cause asthmatic symptoms.
Glutathion-S-transferase (GST) has been considered to be important in the protection of cells from ROS by utilizing oxidative products as a substrate8). GSTs can be categorized into five cytosolic isoforms based on their biochemical, immunologic and structural properties: GST-alpha (GSTA), -mu (GSTM), pi (GSTP), -theta (GSTT) and sigma (GSTS)8). Among them, the GSTP1 gene is located on chromosome 11q139-11) which is an asthma related locus12). Genetic polymorphism of the GSTP1 gene has been shown to be strongly associated with asthma13) and asthma related phenotypes such as atopy and bronchial hyperresponsiveness (BHR)7, 14). Moreover, it has been reported that a Valine (Val) to Isoleucine (Ile) exchange at codon 105 in exon 5 may protect from developing asthma15, 16). In this study, we investigated whether GSTP1 Ile105Val polymorphism may be associated with a susceptibility to AIA phenotype in the Korean population.
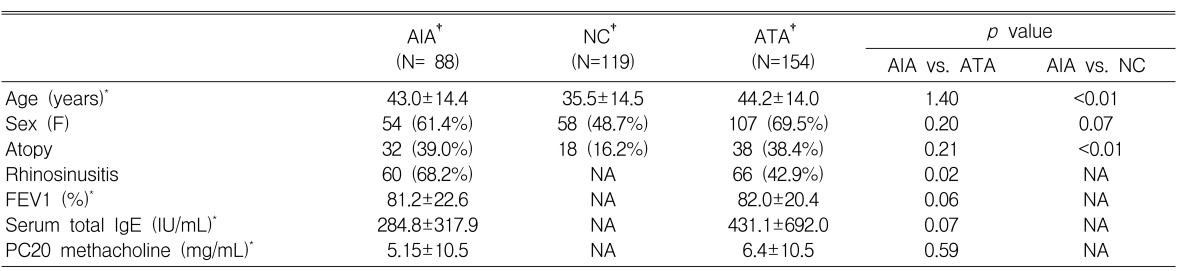
The study population consisted of 88 AIA, 154 aspirin-tolerant asthma (ATA), and 119 normal control (NC) subjects who visited the Allergy Clinic at Ajou University Hospital, Soonchunhyang University Seoul Hospital and Buchon Hospital, in Korea. Diagnosis of AIA was made based on positive responses to a lysine aspirin (L-ASA) bronchoprovocation test which was performed according to a modified method as previously described17). Skin prick tests were performed using 12 common aeroallergens (Bencard, UK), histamine (positive control) and saline (negative control). Atopy was defined as a reactor to one or more common inhalant allergens on skin prick test. All clinical histories were reviewed in detail by the investigator.
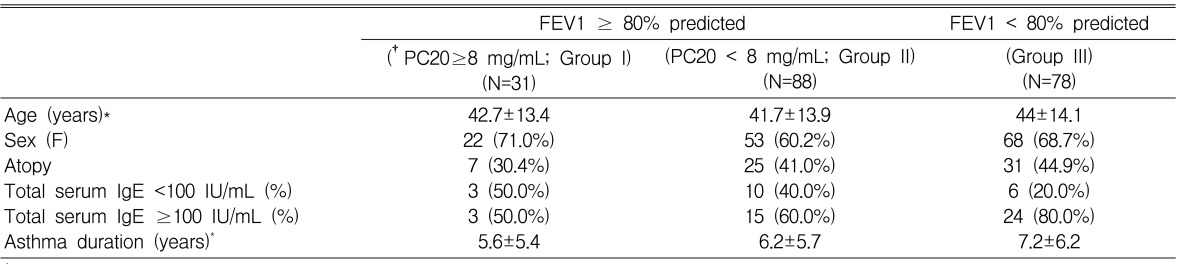
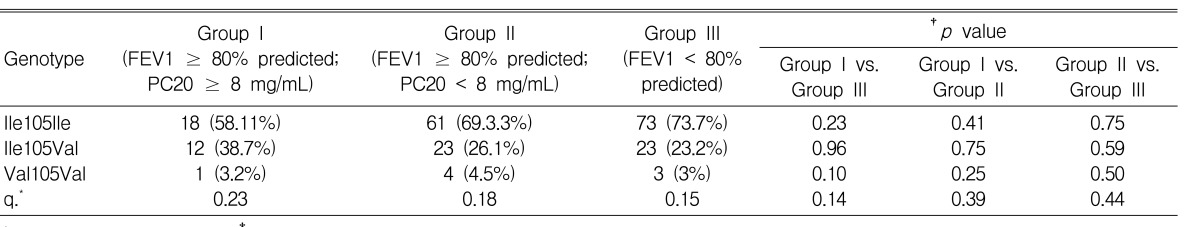
Clinical characteristics of this study are described in Table 1. Bronchial hyperresponsiveness was assessed by the methacholine bronchial challenge test in all subjects. Asthmatic subjects were divided into three groups according to their degree of airway dysfunction as follows: Group I: FEV1Ōēź80% predicted and PC20 methacholine Ōēź 8 mg/mL; Group II: FEV1 Ōēź 80% predicted and PC20 to methacholine < 8 mg/mL; Group III: FEV1 < 80% predicted (Table 3)7, 18).
The exon 5 region of the GSTP1 gene was amplified by primers 5'-TATGGGAAGGACCAGCAGGAG-3' and 5'-CTGCACCCTGACCCAAGAAG-3'. PCR was performed in a mixture containing 1.25 pmol of each primer, 20 ng genomic DNA, 250 uM dNTPs and 0.15U Taq polymerase (Applied Biosystems, Foster City, CA) in the buffer provided by the manufacturer. Amplification was performed in a GeneAmp PCR System 9700 thermal cycler (Applied Biosystems, Foster City, CA). The extension primer (AATCAATGATGATaggacctccgctgcaaatac) was designed for single-base extension (SBE). The primer extension reaction was performed using the SNaPshot ddNTP Primer Extension Kit (Applied Biosystems, Foster City, CA) following the manufacturer directions. To clean up the primer extension reaction products, one unit SAP (Shrimp alkaline phosphate) was added to the reaction mixture and the mixture was then incubated at 37Ōäā for 1h followed by 15min of enzyme inactivation at 72Ōäā.
The difference in allele and genotype frequencies of the GSTP1 Ile105Val polymorphism between the AIA group and the other control groups were analyzed using the Chi-square and Fisher's two-sided exact test. Differences in the mean value of the phenotypic characteristics within the asthmatic subjects were compared using ANOVA test and t-test. All statistical analysis was performed using SPSS Version 11.5 (Chicago, Illinois, USA). A p value of <0.05 was considered to be statistically significant.
Clinical characteristics of the subjects are summarized in Table 1. There were significant differences in mean age and atopy prevalence between AIA and NC groups (p<0.01). The prevalence of rhinosinusitis was significantly higher in the AIA group than in the ATA group (p=0.02). No significant differences in sex, mean age, PC20 methacholine, and basal FEV1 % values were noted between the AIA and ATA groups (p>0.05).
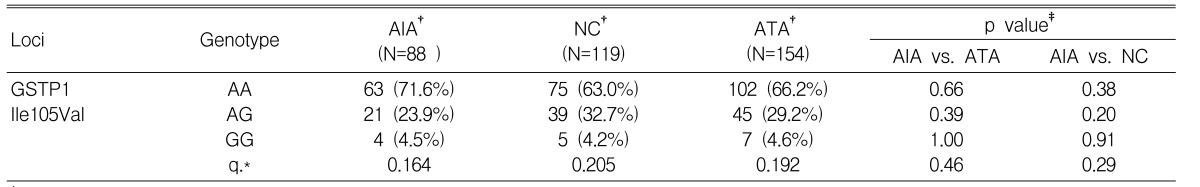
There were no significant differences in allele and genotype frequencies of the GSTP1 Ile105Val polymorphism among the three groups (p>0.05) (Table 2). However, minor G allele frequency of the GSTP1 Ile105Val polymorphism in the AIA group (16.4%) tended to be lower than those of the control groups, ATA (19.2%) and NC (20.5%) (Table 2). Asthma related phenotypes such as atopy, total serum IgE level, predicted basal FEV1% and the PC20 methacholine values were not associated with the GSTP1 Ile105Val polymorphism (p>0.05, data not shown).
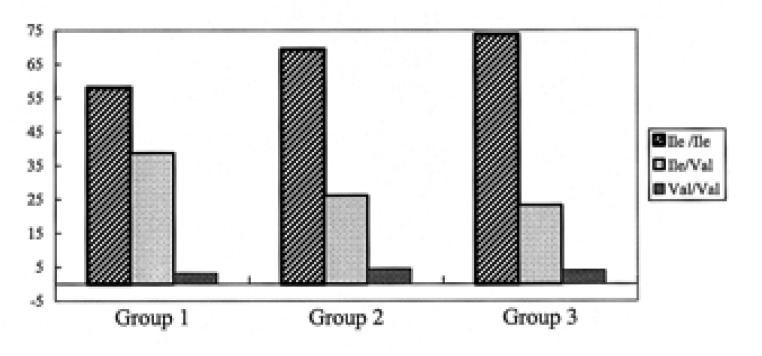
Clinical characteristics of the asthmatic subjects are summarized in Table 3. There were no significant differences among the three groups (p>0.05). Although no statistically significant differences were observed, the duration of asthma for group III subjects (7.2┬▒6.2 years) tended to be longer than for group I (5.6┬▒5.4 years). The allele and genotype frequencies of the GSTP1 Ile105Val polymorphism are shown in Table 4. The proportion of subjects with GSTP1 Ile105Ile in group III tended to be higher than those in groups II and I, whereas the proportion of GSTP1Val105Val displayed an inverse trend (Table 4, Figure 1). Similarly, allele frequency for GSTP1 Val105Val tended to be lower in groups II and III (18.0% and 15.0%, respectively) than in group I (23.0%).
There have been several studies indicating GSTP1 Ile105Val is associated with asthma and its related phenotypes such as atopy and airway hyperresponsiveness to methacholine7, 14). Based on the previous findings that GSTP1 could be involve in the mobilization and regulation of LT release7), we hypothesized that the GSTP1 Ile105Val polymorphism might contribute to AIA pathogenesis and investigated the GSTP1 Ile105Val polymorphism in AIA subjects and compared their results to ATA and NC subjects. In this study, although the frequency of minor G allele of the GSTP1 Ile105Val polymorphism tended to be lower in the AIA group (0.164) than in the other control groups (0.205 for NC, 0.192 for ATA), no statistically significant association was observed between GSTP1 Ile105Val polymorphism and the AIA phenotype. Moreover, no association between asthma related phenotypes such as atopy and airway hyperresponsiveness of the AIA group was observed. Further studies are needed to verify these findings in a larger AIA cohort and non-Korean population.
Regarding the association between the GSTP1 Ile105Val polymorphism and asthma genetics in other populations, the frequency of the GSTP1 Ile105Ile polymorphism might be increased in patients with allergic asthma and chronic obstructive pulmonary disease19). However, studies of other diseases such as rheumatoid arthritis20) and basal cell carcinoma21) suggested that the GSTP1 Ile105Ile polymorphism might have a protective role or the Val105Val polymorphism might be associated with a worse prognosis. Previous studies have suggested that the rare allele frequencies of the GSTP1 Val105Val polymorphism are different based on ethnic population: Asian population (Japanese: 0.14, Taiwanese: 0.18) had a lower Val105 allele frequency compared to African-American (0.42) or European-American (0.33) populations, within the asthmatic cohorts19, 22, 23). In this study the allele frequency of the GSTP1 Val105Val in healthy control subjects was similar to Taiwanese subjects.
Asthma is characterized by airway inflammation12). Accordingly, we also investigated GSTP1 polymorphism according to the degree of airway dysfunction within the asthmatic group. We selected a concentration of 8 mg/mL of PC20 methacholine as the cut-off point for current asthma following standards outlined in other studies7, 18, 24). The frequency of GSTP1 Val105Val allele tended to be lower in subjects with severe airway dysfunction (Group III, 15.0%) compared to subjects with mild airway dysfunction (Group I, 23.0%; Table 4, Figure 1). This result may imply that the GSTP1 Val105Val polymorphism may be related to the progression of airway hyperresponsiveness, which may be comparable to the previous study suggesting that the GSTP1 Val105Val polymorphism was significantly associated with a reduced risk of airway responsiveness and atopy within the allergic asthma group14). The discrepancy in this study may be associated with ethnic or phenotypic differences because AIA is a distinct syndrome differing from ASA tolerant or allergic asthma. Further studies are needed to replicate these association studies in non-Korean populations.
In conclusion, the GSTPI Ilel105Val gene polymorphism was not significantly associated with the AIA phenotype in the Korean population.
Notes
This study was supported by a grant from the Korea Health 21 R&D project, Ministry of Health & Welfare, R.O.K (01-PJ3-PG6-01GN04-0003).
References
1. Quiralte J, Blanco C, Castillo R, Delgado J, Carillo T. Intolerance to non-steroidal anti-inflammatory drugs: results of controlled drug challenges in 98 patients. J Allergy Clin Immunol 1996;98:678ŌĆō685PMID : 8828546.


2. Cowburn AS, Sladek K, Soja J, Adamek L, Nizankowska E, Szczeklik A, Lam BK, Penrose JF, Austen FK, Holgate ST, Sampson AP. Overexpression of leukotriene C4 synthase in bronchial biopsies from patients with aspirin-intolerant asthma. J Clin Invest 1998;101:834ŌĆō846PMID : 9466979.



3. Nasser SM, Pfister R, Christie PE, Sousa AR, Barker J, Schmitz-Schumann M, Lee TH. Inflammatory cell populations in bronchial biopsies from aspirin-sensitive asthmatic subjects. Am J Respir Crit Care Med 1996;153:90ŌĆō96PMID : 8542168.


4. Nasser S, Christie PE, Pfister R, Sousa AR, Walls A, Schmitz-Schumann M, Lee TH. Effect of endobronchial aspirin challenge on inflammatory cells in bronchial biopsy samples from aspirin sensitive asthmatic subjects. Thorax 1996;51:64ŌĆō70PMID : 8658372.



5. Sanak M, Simon HU, Szceklik A. Leukotriene C4 synthase promoter polymorphism and risk of aspirin-induced asthma. Lancet 1997;350:1599ŌĆō1600PMID : 9393345.


6. Barnes PJ. Reactive oxygen species and airway inflammation. Free Radic Biol Med 1990;9:235ŌĆō243PMID : 2272532.


7. Spiteri MA, Bianco A, Strange RC, Fryer AA. Polymorphisms at the glutathione S-transferase, GSTP1 locus: a novel mechanism for susceptibility and development of atopic airway inflammation. Allergy 2000;55:15ŌĆō20PMID : 10919500.

8. Hayes JD, Strange RC. Potential contribution of the glutathione S-transferase supergene family to resistance to oxidative stress. Free Radic Res 1995;22:193ŌĆō207PMID : 7757196.


9. Thomas NS, Wilkinson J, Holgate ST. The candidate region approach to the genetics of asthma and allergy. Am J Respir Crit Care Med 1997;156:S144ŌĆōS151PMID : 9351596.


10. The Collaborative Study on the Genetics of Asthma. A genomewide search for asthma susceptibility loci in ethnically diverse populations. Nat Genet 1997;15:389ŌĆō392PMID : 9090385.



11. Daniels SE, Bhattacharrya S, James A, Leaves NI, Young A, Hill MR, Faux JA, Ryan GF, le Souef PN, Lathrop GM, Musk AW, Cookson WO. A genome-wide search for quantitative trait loci underlying asthma. Nature 1996;383:247ŌĆō250PMID : 8805698.


12. Doull IJ, Lawrence S, Watson M, Begishvili T, Beasley RW, Lampe F, Holgate T, Morton NE. Allelic association of gene markers on chromosome 5q and 11q with atopy and bronchial hyperresponsiveness. Am J Respir Crit Care Med 1996;153:1280ŌĆō1284PMID : 8616554.


13. Fryer AA, Bianco A, Hepple M, Jones PW, Strange RC, Spiteri MA. Polymorphism at the glutathione S-transferase GSTP1 locus: a new marker for bronchial hyperresponsiveness and asthma. Am J Respir Crit Care Med 2000;161:1437ŌĆō1442PMID : 10806136.


14. Mapp CE, Fryer AA, de Marzo N, Pozzato V, Padoan M, Boschetto P, Strange RC, Hemmingsen A, Spiteri MA. Glutathione S-transferase GSTP1 is a susceptibility gene for occupational asthma induced by isocyanates. J Allergy Clin Immunol 2002;109:867ŌĆō872PMID : 11994713.


15. Garcia-Saez I, Parraga A, Phillips MF, Mantle TJ, Coll M. Molecular structure at 1.8 A of mouse liver class pi glutathione S-transferase complexed with S-(p-nitrobenzyl)glutathione and other inhibitors. J Mol Biol 1994;237:298ŌĆō314PMID : 8145243.


16. Zimniak P, Nanduri B, Pikula S, Bandorowicz-Pikula J, Singhal SS, Srivastava SK, Awasthi S, Awasthi YC. Naturally occurring human glutathione S-transferase GSTP1-1 isoforms with isoleucine and valine in position 104 differ in enzymic properties. Eur J Biochem 1994;224:893ŌĆō899PMID : 7925413.


17. Choi JH, Park HS, Oh HB, Lee JH, Suh YJ, Park CS, Shin HD. Lekotriene related gene polymorphisms in ASA-intolerant asthma: an
association with a haplotype of 5-lipoxygense. Hum Genet 2004;114:337ŌĆō344PMID : 14749922.


18. Cameron AD, Sinning I, L'Hermit G, Olin B, Board PG, Mannervik B, Jones TA. Structural analysis of human alpha-class glutathione transferase A1-1 in the apo-form and in complexes with ethacrynic acid and its glutathione conjugate. Structure 1995;3:717ŌĆō727PMID : 8591048.


19. Stephens EA, Taylor JA, Kaplan N, Yang CH, Hsieh LL, Lucier GW, Bell DA. Ethnic variation in the CYP2E1 gene: polymorphism analysis of 695 African-Americans, European-Americans and Taiwanese. Pharmacogenetics 1994;4:185ŌĆō192PMID : 7987402.


20. Mattey DL, Hassell AB, Plant M, Dawes PT, Ollier WR, Jones PW, Fryer AA, Alldersea JE, Strange RC. Association of polymorphism in glutathione S-transferase loci with susceptibility and outcome in rheumatoid arthritis: comparison with the shared epitope. Ann Rheum Dis 1999;58:164ŌĆō168PMID : 10364914.



21. Ramachandran S, Hoban P, Ichii-Jones F, Pleasants L, ali-Osman F, Lear J, Smith AG, Bowers B, Jones PW, Fryer AA, Strange RC. Glutathione S-transferase GSTP1 and cyclin D1 genotypes: associations with numbers of basal cell carcinomas in a patient subgroup at high-risk of multiple tumors. Pharmacogenetics 2000;10:545ŌĆō556PMID : 10975609.


22. Watson MA, Stewart RK, Smith GB, Massey TE, Bell DA. Human glutathione S-transferase P1 polymorphisms: relationship to lung tissue enzyme activity and population frequency distribution. Carcinogenesis 1998;19:275ŌĆō280PMID : 9498276.


Figure┬Ā1
Association of the GSTP1 Ile105Val polymorphism with airway dysfunction. Subjects were divided into three groups according to their airway dysfunction: group I: FEV1 Ōēź 80% predicted and PC20 Ōēź 8 mg/mL; group II: FEV1 Ōēź 80% predicted and PC20 < 8 mg/mL; group III: FEV1< 80% predicted.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



