 |
 |
| Korean J Intern Med > Volume 21(3); 2006 > Article |
|
Abstract
Photodynamic therapy (PDT) that is based on the science of photochemistry has been recognized as a lung sparing local therapeutic modality that can achieve remarkable responses. It is an alternative treatment for early stage lung cancer patients who have poor lung function or multiple sites of cancer. Recently we treated a 70-year-old man who presented with squamous carcinoma
in situ at a previous pneumonectomy site, and a 64-year-old man with a newly developed secondary superficial lung cancer, with PDT. There were no complications related to the procedure. Both patients had poor lung function due to prior lung cancer surgery. Clinical and histological complete remissions were achieved without any evidence of recurrence during 30 months of follow-up in both patients.
Photodynamic therapy (PDT) is treatment with drugs that react to light; the mechanisms of action is based on the science of photochemistry. The effects of PDT are based on the interaction of a tumor-selective photosensitizer and a laser beam of light. This interaction causes selective death of tumor cells. A basic photochemical reaction occurs when a sensitizer in an excited state in turn interacts with molecular oxygen to form a singlet oxygen or oxygen-free radicals. Singlet oxygen, the main product of a photochemical reaction, is a powerful oxidizing agent. The majority of clinical data using PDT, in early lung cancer, has been obtained from the treatment of patients who were not surgical candidates. The majority of clinical studies have emerged from Japan over the last two decades1-3). PDT has been shown to yield high rates of complete responses (CR) in patients with an early stage central type of lung cancer. PDT is a good alternative for patients with poor lung function or multiple sites of cancer. In Korea, there has been only one report on the palliative use of PDT4), and no reports on its use for early lung cancer. Therefore we present our experience with PDT used successfully for early superficial lung carcinoma.
A 70-year-old Korean man presented with squamous carcinoma
in situ at, a previously treated, left main anastomotic pneumonectomy site. A left pneumonectomy was performed for squamous lung cancer 20 months previously; at that time the surgical margin was positive for squamous cell carcinoma. The patient was treated with concurrent chemoradiotherapy with six cycles of weekly paclitaxel (45 mg/m2) plus cisplatin (20 mg/m2) during radiotherapy with 5,040 cGy, and received four cycles of consolidation chemotherapy by paclitaxel plus cisplatin every three weeks. Follow-up bronchoscopic findings, after 20 months, showed that the mucosa in the anastomotic site had progressed with hypervascular infiltrative change; a biopsy revealed progression of cancerous changes. Bronchoscopic PDT was performed under local anesthesia with conscious sedation (Midazolam 2 mg intravenous injection). Before the PDT procedure, the photosensitizer (Photogem®: Lomonosov Institute of Fine Chemical, Russia) was injected intravenously at a dose of 2.0 mg/kg body weight. After an injection of Photogem, the patients were instructed to avoid direct sunlight exposure for at least two weeks. The laser beam was transmitted via a quarz fiber (400 mm) inserted through the instrumentation channel of a fiberoptic bronchoscope. A light using Diode laser (Biolitec Inc., Germany, wavelength; 633 nm) was delivered to the anastomotic site at 200 J/cm2 with a 3 cm diffuser and 100 J/cm2 with a 2 cm diffuser for 500 seconds, respectively. Fourty-eight hours after the procedure, bronchial toilet was performed. Necrotic changes at the PDT treated site were noted. There were no immediate complications. We performed a follow up bronchoscopic biopsy after three and 12 months. The histology at the PDT site regressed to focal squamous metaplasia and inflammatory conversion (Figure 1). Clinical and histological findings were consistent with complete remission; there was no evidence of recurrence during 30 months of follow-up.
A 64-year-old Korean man was treated for squamous lung cancer IIIa (T1N2M0) 26 months prior to presentation; he now presented with subepithelial stroma that contained a very tiny nest of squamous cell carcinoma cells in the left upper lobe bronchus. The patient previously, 26 months ago, had surgery to remove the right upper and middle lobes of the lung; the patient received six cycles of adjuvant chemotherapy and chest radiation therapy with 5,520 cGy. We provided PDT at the left upper bronchus with 200J and a 2 cm diffuser for 400 seconds. After 48 hours, there were no necrotic conversions observed but edematous hyperemic mucosal changes were present. At eight months after the first PDT, a nodular mass was noted at the left upper apical segment. The pathology results from a bronchoscopic biopsy identified a squamous cell carcinoma. Light was delivered at 250 J/cm2 with a diffuser length for 625 seconds, and 100 J/cm2 for 250 seconds at the same site and again after 48 hours, respectively. After PDT, there were no immediate complications such as hemoptysis or respiratory failure. We performed a follow up bronchoscopy at one and four months. The previous cancer site showed inflammatory conversion; there was no remnant tumor tissue (Figure 2). The patient is alive and well at 30 month follow up.
It has been known for nearly a century that a chemical reaction occurs when photosensitizing compounds are exposed to light. In 1961 Lipson showed that hematoporphyrin, prepared from hematoprophyrin hydrochloride, and by treatment with acetic acid had a great affinity for malignant tissue5). PDT first reported by Dougherty et al.6) has attracted increasing interest worldwide. Hayata et al.1) reported the result of complete tumor remission in early superficial lung cancer. Since then there have been many reports on the results of PDT for early lung cancer.
Keto et al.2) has reported that one hundred forty-five patients (191 cancers), with early non-small cell lung cancer, have been treated with PDT since 1980. The majority of cases (98%) were squamous cell carcinoma. CR was achieved in 86% of lesions with a recurrence rate of 13%, resulting in a long-term response of 75%. When success of treatment was evaluated according to lesion size, lesions < 1.0 cm had a CR of 95%, and lesions 2 cm had a CR of only 46%. Ono et al.7) studied 36 patients (39 cancers) and achieved a CR of only 31%, with a recurrence in 33%. Therefore, the long-term response was only 21%. Lam et al.8) reported 102 patients with radiologically occult (stage 0, IA, and IB) squamous cell lung cancer treated with PDT. An overall immediate CR of 78% was achieved. Forty-four percent of the patients had recurrent tumor on follow-up, giving a long-term response rate of 43%. The median time to tumor recurrence was 2.8 years. The Mayo Clinic has reported treatment of 58 non-surgical patients with early lung cancer with an 84% CR rate achieved after one treatment9). Nineteen patients (39%) with recurrent tumor had a second PDT treatment. The median time to tumor recurrence after the first treatment was 4.1 years. Following the second treatment, 11 patients (22%) had recurrences. The long-term CR rate was 66%.
Experience remains limited using PDT for patients who are surgical candidates. PDT as an alternative to surgical resection was studied in 21 patients with small bronchial cancer10). A 71% of CR (15 of 21 patients) was achieved, with 11 patients (52%) maintaining a CR > 12 months. Patients who did not respond or had a recurrence were offered surgery. Of the 10 patients who underwent surgery, three patients were found to have N1 disease. Two patients refused surgery. A total of nine patients (43%) were spared surgery. For patients with early superficial squamous cell carcinoma who are surgical candidates, the use of PDT appears to be a promising treatment, but more experience is needed to compare PDT to surgical outcomes.
PDT is also approved for palliation of malignant endobronchial obstruction. The response of PDT is not dependent on the tumor cell type. It can be applied in patients who have already undergone surgery, radiation, or chemotherapy. Penetration of PDT is limited to five to 10 mm from the tissue surface. In cases with an invading tumor more than 1cm, it might be difficult to manage with PDT alone. The effectiveness of PDT, for symptom palliation and survival, has been evaluated in patients with advanced inoperable bronchogenic cancer and endobronchial obstruction. Moghissi et al11) reported on 100 patients; 82% had received prior chemotherapy and/or radiotherapy, diminished endoluminal obstruction was achieved in 86 to 18% after PDT. This study suggested that PDT is an effective technique for palliation of inoperable advanced lung cancer in a subset of patients.
Electrocautery is a less expensive treatment approach for endobronchial tumors. A small study of early lung cancer in 13 patients (15 cancers) showed a CR of 80% in lesions with no recurrence at 22 months follow-up12). Cryotherapy is a technique used where tissue is destroyed by freezing; it is the least expensive method available for treatment. Deygas et al13) reported on 35 patients (41 cancers) with early stage lung cancer. A CR was obtained in 91% of the patients with a recurrence rate of 28% within four years. A long-term response rate of 63% was achieved, similar to that of PDT. Brachytherapy refers to the placement of a radioactive source within or near an endobronchial malignancy to deliver local irradiation. Perol et al14) reported on 34 patients with early stage lung cancer with a CR of 85% observed over two years of follow-up. The use of Nd-YAG laser treatment for early lung cancer has not been widely studied to date.
Following PDT treatment, patients should be closely monitored for recurrent disease and for the development of metachronous lesions. These patients should undergo bronchoscopic examination every three to six months with both white light and fluorescence bronchoscopy, if available. PDT is contraindicated for patients with: critical central airway obstruction because of the delay of improvement, for tumors invading the esophagus or major vessels, and for patients with porphyria or an allergy to components of the photosensitizer, in addition to the general contraindications for rigid or flexible bronchoscopy. The most common complication of photodynamic therapy is skin photosensitivity. This may last for up to eight weeks after injection of the photosensitizer. All patients receiving these agents must take careful precautions to avoid significant light exposure during the period of sensitivity. Local complications from the treatment include airway edema, necrosis and stricture. Tumor lysis can result in a bronchovascular or tracheoesophageal fistula. Fatal hemoptysis has been reported, but its relationship to photodynamic therapy, as opposed to the progression of disease alone, is unclear15).
In summary, we present two cases of early superficial endobronchial cancer treated with PDT. This procedure was safe and effective for the treatment and management of early superficial squamous cell carcinoma.
References
1. Hayata Y, Kato H, Konaka C, Ono J, Takizawa N. Hematoporphyrin derivative and laser photoradiation in the treatment of lung cancer. Chest 1982;81:269–277PMID : 6276108.


2. Kato H, Harada M, Ichinose S, Usuda J, Tsuchida T, Okunnaka T. Photodynamic therapy (PDT) of lung cancer: experience of the Tokyo Medical University. Photodiagnosis and Photodynamic Therapy 2004;1:49–55.


3. Imamura S, Kusunoki Y, Takifuji N, Kudo S, Matsui K, Masuda N, Takada M, Ryu S, Fukuoka M. Photodynamic therapy and/or external beam radiation therapy for roentgenologically occult lung cancer. Cancer 1994;73:1608–1614PMID : 8156487.


4. Yoon SH, Han KT, Kim GN, Lee SI. Effect of photodynamic therapy in lung cancer. Tuberc Respir Dis 2004;57:358–363.

5. Lipson RL, Baldes EJ, Olsen AM. The use of a derivative of hematoporphyrin in tumor detection. J Natl Cancer Inst 1961;26:1–11PMID : 13762612.

6. Dougherty TJ, Kafman JE, Goldfarb A, Weishaut KR, Boyle D, Mittleman A. Photoradiation therapy for the treatment of malignant tumors. Cancer Res 1978;38:2628–2635PMID : 667856.

7. Ono R, Ikeda S, Suemasu K. Hematoporphyrin derivative photodynamic therapy in roentgenographically occult carcinoma of the tracheobronchial tree. Cancer 1992;69:1696–1701PMID : 1532341.


8. Lam S. Bronchoscopic, photodynamic, and laser diagnosis and therapy of lung neoplasms. Curr Opin Pulm Med 1996;2:271–276PMID : 9363151.

9. Cortese DA, Edell ES, Kinsey JH. Photodynamic therapy for early stage squamous cell carcinoma of the lung. Mayo Clin Proc 1997;72:595–602PMID : 9212759.


10. Edell ES, Cortese DA. Photodynamic therapy: treatment of early stage lung cancer: results and follow-up. Excerpta Medica International Congress series 1990;Vol. 89:205–210.
11. Moghissi K, Dixon K, Stringer M, Freeman T, Thorpe A, Brown S. The place of bronchoscopic photodynamic therapy in advanced unresectable lung cancer: experience of 100 cases. Eur J Cardiothorac Surg 1999;15:1–6PMID : 10077365.


12. van Boxem TJ, Venmans BJ, Schramel FM, van Mourik JC, Golding RP, Postmus PE, Sutedja TG. Radiographically occult lung cancer treated with fiberoptic bronchoscopic electrocautery: a pilot study of a simple and inexpensive technique. Eur Respir J 1998;11:169–172PMID : 9543288.


13. Deygas N, Froudarakis M, Ozenne G, Vergnon JM. Cryotherapy in early superficial bronchogenic carcinoma. Chest 2001;120:26–31PMID : 11451811.


Figure 1
Bronchoscopic and pathologic changes from Case 1 before and after photodynamic therapy. (A) Bronchoscopic finding before treatment (2001.12.27). (B) Squamous cell carcinoma (H&E ×200) in carina. (C) Bronchoscopic findings after concurrent chemoradiotherapy (2002.4.1). (D) Focal metaplasia (H&E ×200). (E) Before photodynamic therapy (PDT) (2003. 7.15). (F) Squamous cell carcinoma (H&E ×100). (G) Four days after PDT (2003.7.28). (H) Necrotic tissue (H&E ×40). (I) Eight months after PDT (2004. 4.13). (J) Focal squamous metaplasia (H&E ×40). (K) One year after PDT (2004. 7.29). (L) Non-specific bronchitis (H&E ×100)
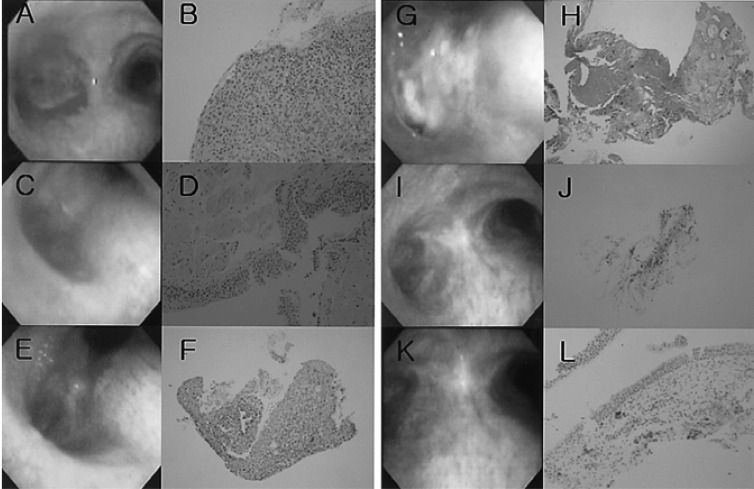
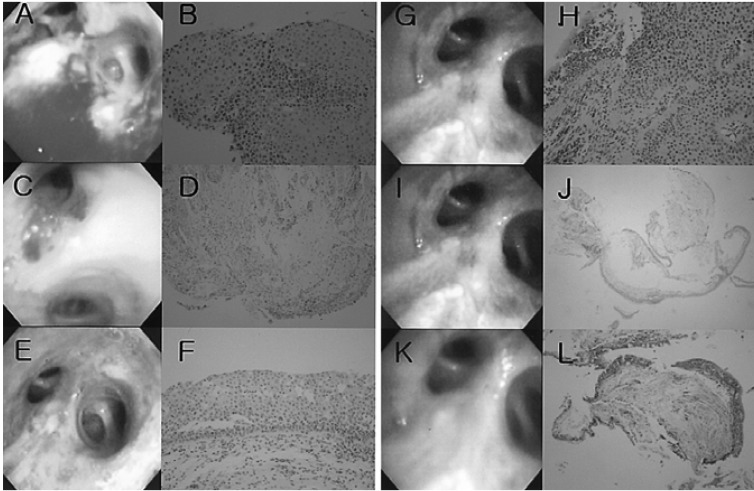
Figure 2
Bronchoscopic and pathologic changes of Case 2 before and after photodynamic therapy. (A) Bronchoscopic findings before photodynamic therapy (PDT) (2003.7.18). (B) Squamous cell carcinoma in the left upper lobe bronchus (H&E ×200). (C) Four days after first PDT (2003.7.28). (D) Edematous change (H&E ×100). (E) Five months after first PDT (2003. 12.8). (F) Low grade dysplasia (H&E ×200). (G) Recurrent mass after eight months (2004.3.9). (H) Squamous cell carcinoma. (H&E ×200). (I) One month after second PDT (2004.3.13). (J) Non-specific bronchitis (H&E ×40). (K) Five months after second PDT (2004. 7.19). (L) Non-specific bronchitis (H&E ×40).



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



