 |
 |
| Korean J Intern Med > Volume 21(2); 2006 > Article |
|
Abstract
Background
To assess the efficacy of fenofibrate treatment in combination with urate lowering agents in patients with gout.
Methods
Fourteen male patients with chronic tophaceous or recurrent acute attacks of gout were evaluated in an open-label pilot study of the hypolipidemic agent, fenofibrate (Lipidil Supra┬« 160 mg/d). Patients were stable on urate lowering agents (allopurinol or benzbromarone) for Ōēźthree months without acute attack for the most recent one month before participating. All patients were being treated with established doses of urate lowering agents without modification throughout the study. Clinical and biochemical assessments including serum uric acid, creatinine, liver function test and fasting serum lipid were measured at (1) baseline (2) after two months of fenofibrate treatment and (3) two months after fenofibrate was withdrawn.
Results
Serum uric acid was lowered by 23% after two months of fenofibrate treatment (6.93┬▒2.16 vs. 5.22┬▒1.16 mg/dL; p=0.016). Triglyceride levels were also reduced after fenofibrate treatment (p=0.001). However, this effect was reversed after the withdrawal (p=0.002) of the drug. Alkaline phosphatase was reduced after fenofibrate treatment (p=0.006), but increased 21% after the withdrawal of the drug (p=0.002). By contrast, serum levels of high density lipoprotein and creatinine were increased 9% (p=0.018) and 12% (p=0.006), respectively; however, both levels were significantly decreased to the baseline levels upon withdrawal of fenofibrate.
Gout is the most common cause of inflammatory arthritis in middle aged men; it has been reported to have a prevalence of 2.3 to 41.4%, in a variety of populations1). The incidence of gout in Asian populations has been reported to be lower than in Western population2, 3). In recent decades, with changes in dietary habits, the overall prevalence of gout in Asian populations is steadily increasing4, 5).
Gout is a metabolic syndrome associated with by hyperlipidemia, hypertension, and insulin resistance as well as hyperuricemia. Hypertriglyceridemia is a frequent lipid abnormality found in patients with gout. Approximately 75~80% of the patients with gout have coexisting hypertriglyceridemia6). Hyperuricemia is also found in a considerable number of patients with hypertriglyceridemia6). However, drugs currently in use to treat gout, including allopurinol and benzbromarone, are effective only in correcting the underlying hyperuricemia, not the other coexisting metabolic abnormalities. In addition, the choice of anti-hyperuricemic agents is very limited; this limits optimal gout management in current clinical practice. Therefore, new therapeutic options for treatment of gout are needed, especially for co-targeting associated metabolic abnormalities.
Fenofibrate is a fibric acid derivative that has an established effect; it has been shown to reduce total cholesterol, triglyceride, LDL- and VLDL-cholesterol7). Recently, it has been reported that fenofibrate can also reduce serum uric acid8-13). The purpose of this study was to assess the clinical and biochemical effects of fenofibrate treatment, in combination with urate lowering agents, in patients with gout.
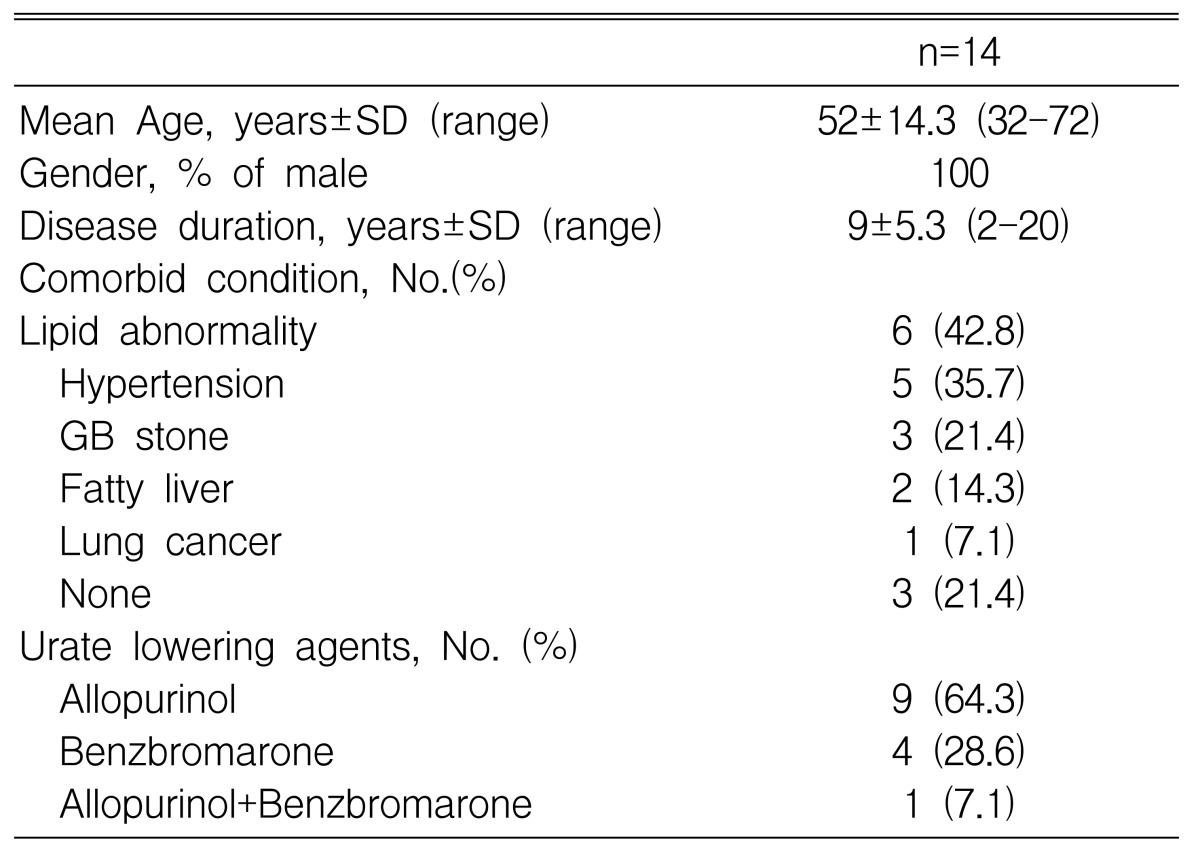
Fourteen male patients, with a history of recurrent acute or chronic tophaceous gout were recruited for this study. Each patient had been receiving allopurinol or benzbromarone for at least three months, and had been free from acute gout for a minimum of one month prior to participating in the study. Although six patients also had hyperlipidemia (hypercholesterolemia and/or hypertriglyceridemia) upon entering the study, they were not being treated with lipid lowering agents. Demographic data for the study group are presented in Table 1. The mean age of the patients was 52 yrs (range 32-72) with a disease duration of 9┬▒5.3 yrs (range 2-20). Co-morbid conditions found in the study population were: hypertension in five patients, lipid abnormality in six , gallstones in three , fatty liver in two, and lung cancer in one. The urate lowering agents used for treatment were allopurinol in nine patients, benzbromarone in four, and both in one.
The study design followed an open-label pilot protocol. Clinical and biochemical assessments were performed at (1) baseline (2) after two months of added fenofibrate and (3) two months after fenofibrate was withdrawn. Fenofibrate (Lipidil Supra®) 160 mg was given daily. Patients were monitored for the development of an acute gout attack. Biochemical parameters including: serum urate, lipid profile (total cholesterol, triglyceride, HDL, and LDL cholesterol), liver function tests (aspartate transaminase, alanine aminotransferase, and alkaline phosphatase), and renal function tests (BUN and creatinine) were evaluated with a Hitachi 7600 analyzer. The established daily doses of urate lowering agents were continued during fenofibrate treatment, and after its withdrawal. Prophylaxis against acute flares of gout was not routinely provided. Patients were instructed to make minimal dietary and lifestyle changes during the study period.
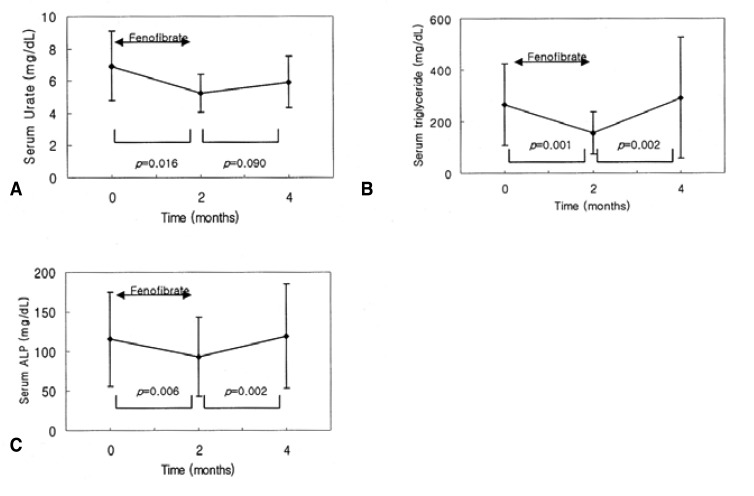
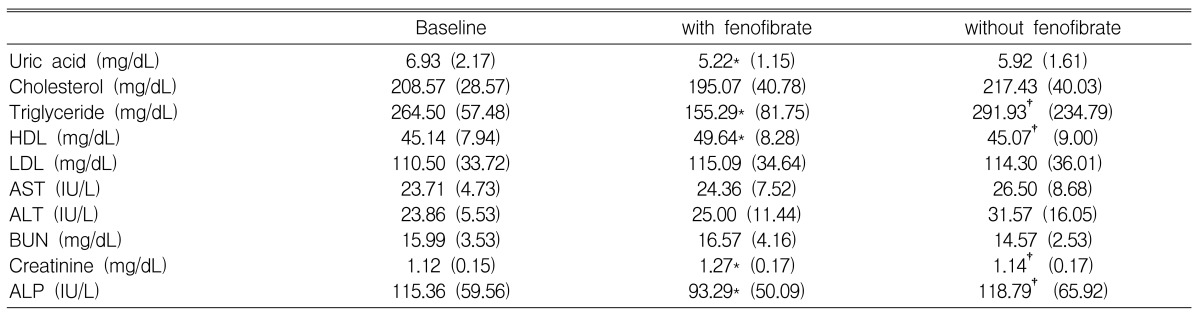
Serum uric acid was significantly lowered, by 23%, after two months of fenofibrate treatment (6.93┬▒2.16 vs. 5.22┬▒1.16 mg/dL; p=0.016). The uric acid level showed a tendency to increase after fenofibrate withdrawal; however this difference did not reach statistical significance (5.22┬▒1.16 vs. 5.92┬▒1.61 mg/dL; p=0.090) (Table 2, Figure 1).
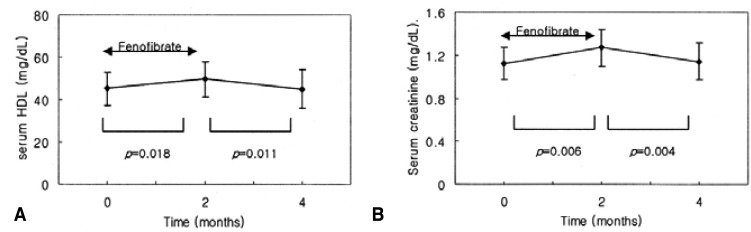
Changes in lipid profiles, with and without fenofibrate treatment, are shown in Table 2. Triglyceride levels were significantly reduced after fenofibrate treatment (264.5┬▒157.48 vs. 155.29┬▒81.75 mg/dL; p=0.001); however, this effect was reversed after the withdrawal of the drug (155.29┬▒81.75 vs. 291.93┬▒234.79 mg/dL; p=0.002) (Figure 1). HDL cholesterol was significantly increased after fenofibrate therapy (45.14┬▒7.94 vs. 49.64┬▒8.28 mg/dL; p=0.018); however, it was reduced after fenofibrate withdrawal (49.64┬▒8.28 vs. 45.07┬▒9.00 mg/dL; p=0.011) (Figure 1). There were no significant changes in total cholesterol and LDL-cholesterol levels with and without fenofibrate therapy.
Alkaline phosphatase (ALP) was reduced after fenofibrate treatment (115.36┬▒59.56 vs. 93.29┬▒50.09 IU/L; p=0.006); however, it was increased after the withdrawal of the drug (93.29┬▒50.09 vs. 118.79┬▒65.92 IU/L; p=0.002) (Figure 2). Serum creatinine was increased after fenofibrate therapy (1.12┬▒0.15 vs. 1.27┬▒0.17 mg/dL; p=0.006); but decreased after the withdrawal of the drug (1.27┬▒0.17 vs. 1.14┬▒0.17 mg/dL; p=0.004) (Figure 2).
Our study confirmed an additional urate lowering effect with fenofibrate in Korean patients with gout who were being treated with anti-hyperuricemic agents. Our study findings showed that serum urate was reduced an additional 23% with fenofibrate therapy. This result is consistent with previous studies on healthy subjects that demonstrated a 23~31% reduction of serum urate after treatment with fenofibrate at six or 12 weeks10, 14). The antihyperuricemic effect of fenofibrate occurred after a relatively short period of time at eight weeks after initiation of fenofibrate treatment; this was reversed after discontinuation of the drug. Compliance with the medication protocol was not a compounding factor in this study since alkaline phosphatase activity was decreased in each patient; this is a well-described effect of fibrate treatment14-18).
The mechanism of action underlying the serum urate lowering effect of fenofibrate is primarily through its uricosuric effects; this causes an increase in the fractional excretion of urate. The fractional excretion of urate is defined as the percent of urate excreted in the urine that is filtered at the glomerulus, which is normally less than 10%19). Since fenofibrate can enhance uricosuric effects, renal stones may be an unpleasant side effect. Therefore, development of renal stones should be monitored closely when fenofibrate is administered, especially in patients receiving benzbromarone. Although the duration of fenofibrate treatment was relatively short, development of renal stones was not noted during the study period. However, we observed a mild but significant increase in the serum creatinine level; this finding suggests caution when using fenofibrate in patients with gout that also have renal insufficiency.
Recently, hyperuricemia has been reported as one of the metabolic abnormalities that can lead to the metabolic syndrome20). Hyperuricemia is frequently associated with hypertriglyceridemia; this can cause the metabolic syndrome in patients with pancreatitis or coronary heart disease. The Apo e4 allele polymorphism, obesity and alcohol consumption have been implicated as causal factors for the metabolic syndrome combined with hyperuricemia and hypertriglyceridemia; however, the mechanisms underlying this are not yet clearly identified21, 22). In addition, it is not clear whether lowering the triglyceride levels or uric acid can improve the prognosis of this combined metabolic syndrome. Dietary reduction of triglyceride intake has been shown to increase the renal excretion of urate, but this effect was minimal and transient23). Since dietary modulations are often very difficult for patients to follow, drugs to control the combined metabolic syndrome with hyperuricemia and hyperlipidemia are needed. We have demonstrated that administration of a standard clinical dose of fenofibrate led to a decrease in the uric acid level, as well as a reduction in triglyceride levels and elevation of HDL cholesterol. The lipid lowering effect of fenofibrate was profound for the triglyceride levels, but less prominent for the total cholesterol and LDL cholesterol. In our study, approximately 40% of the studied patients had a combined lipid abnormality. Since many patients with gout, were also affected by a dyslipidemia the dual lowering effect of fenofibrate on triglyceride levels and uric acid could be extremely beneficial.
It is known that a sudden alteration of serum urate concentrations may lead to the development of an acute attack of gout arthritis. Although fenofibrate lowered serum urate concentrations in a relatively short period of time, there was no acute attack of gout arthritis with fenofibrate treatment. This may be explained in part by the anti-inflammatory property of fenofibrate. Fenofibrate is a PPAR activator which can down-regulate the expression of the inducible COX-2 enzyme, resulting in an anti-inflammatory effect24).
Although fenofibrate was shown to effectively reduce uric acid levels in patients who were already on urate lowering agents, our results are limited by a small study population since it was an open-label pilot study. We can provide more significant evidence with a larger study population.
In conclusion, fenofibrate was effective in providing an additional hypouricemic effect when combined with established urate lowering agents. For gout patients who also have hypertriglyceridemia, fenofibrate treatment can also lower these blood levels. Since choice of antihyperuricemic agents is limited, fenofibrate can offer an additional option for patients with gout.
Notes
This study was supported in part by Ewha Womans University Mokdong Hospital clinical research fund.
References
1. Ghei M, Mihailescu M, Levinson D. Pathogenesis of hyperuricemia: recent advances. Curr Rheumatol Rep 2002;4:270ŌĆō274PMID : 12010614.


2. Dai SM, Han XH, Zhao DB, Shi YQ, Liu Y, Meng JM. Prevalence of rheumatic symptoms, rheumatoid arthritis, ankylosing spondylitis, and gout in Shanghai, China: a COPCORD study. J Rheumatol 2003;30:2245ŌĆō2251PMID : 14528524.

3. Chang HY, Pan WH, Yeh WT, Tsai KS. Hyperuricemia and gout in Taiwan: results from the Nutritional and Health Survey in Taiwan (1993-96). J Rheumatol 2001;28:1640ŌĆō1646PMID : 11469473.

4. Yoo B. Serum uric acid levels in Korean adult population and their correlates. J Korean Rheum Assoc 1995;2:60ŌĆō68.
5. Arromdee E, Michet CJ, Crowson CS, O'Fallon WM, Gabriel SE. Epidemiology of gout: is the incidence rising? J Rheumatol 2002;29:2403ŌĆō2406PMID : 12415600.

6. Takahashi S, Yamamoto T, Moriwaki Y, Tsutsumi Z, Higashino K. Impaired lipoprotein metabolism in patients with primary gout: influence of alcohol intake and body weight. Br J Rheumatol 1994;33:731ŌĆō734PMID : 8055199.


7. Adkins JC, Faulds D. Micronised fenofibrate: a review of its pharmacodynamic properties and clinical efficacy in the management of dyslipidaemia. Drugs 1997;54:615ŌĆō633PMID : 9339964.


8. Desager JP, Hulhoven R, Harvengt C. Uricosuric effect of fenofibrate in healthy volunteers. J Clin Pharmacol 1980;20:560ŌĆō564PMID : 7440763.


9. Feher MD, Hepburn AL, Hogarth MB, Ball SG, Kaye AE. Fenofibrate enhances urate reduction in men treated with allopurinol for hyperuricaemia and gout. Rheumatology 2003;42:321ŌĆō325PMID : 12595630.


10. Noguchi Y, Tatsuno I, Suyama K, Shibata T, Yoshida T, Otsuka Y, Fuse M, Takeo C, Saito Y. Effect of fenofibrate on uric acid metabolism in Japanese hyperlipidemic patients. J Atheroscler Thromb 2004;11:335ŌĆō340PMID : 15644587.


11. Desager JP, Hulhoven R, Harvengt C. Uricosuric effect of fenofibrate in healthy volunteers. J Clin Pharmacol 1980;20:560ŌĆō564PMID : 7440763.


12. Takahashi S, Moriwaki Y, Yamamoto T, Tsutsumi Z, Ka T, Fukuchi M. Effect of combination treatment using anti-hyperuricaemic agents with fenofibrate and/or losartan on uric acid metabolism. Ann Rheum Dis 2003;62:572ŌĆō575PMID : 12759298.



13. Bastow MD, Durrington PN, Ishola M. Hypertriglyceridemia and hyperuricemia: effect of two fibric acid derivatives (bezafibrate and fenofibrate) in a double-blind, placebo-controlled trial. Metabolism 1988;37:217ŌĆō220PMID : 3278190.


14. de la Serna G, Cadarso C. Fenofibrate decreases plasma fibrinogen, improves lipid profile, and reduces uricemia. Clin Pharmacol Ther 1999;66:166ŌĆō172PMID : 10460070.


15. Day AP, Feher MD, Chopra R, Mayne PD. The effect of bezafibrate treatment on serum alkaline phosphatase isoenzyme activities. Metabolism 1993;42:839ŌĆō842PMID : 8102192.


16. Dohmen K, Mizuta T, Nakamuta M, Shimohashi N, Ishibashi H, Yamamoto K. Fenofibrate for patients with asymptomatic primary biliary cirrhosis. World J Gastroenterol 2004;10:894ŌĆō898PMID : 15040040.



17. Ganotakis E, Tsimihodimos V, Bairaktari E, Rizos E, Athyros V, Seferiades C, Elisaf M. Effect of various fibrates on serum alkaline phosphatase activity. Atherosclerosis 2002;165:187ŌĆō188PMID : 12208487.


18. Papadakis JA, Ganotakis ES, Jagroop IA, Winder AF, Mikhailidis DP. Statin+fibrate combination therapy: fluvastatin with bezafibrate or ciprofibrate in high risk patients with vascular disease. Int J Cardiol 1999;69:237ŌĆō244PMID : 10402106.


19. Maesaka JK, Fishbane S. Regulation of renal urate excretion: a critical review. Am J Kidney Dis 1998;32:917ŌĆō933PMID : 9856507.


20. Schmidt MI, Watson RI, Duncan BB, Metcalf P, Brancati FL, Sharrett AR, Davis CE, Heiss G. Clustering of dyslipidemia, hyperuricemia, diabetes, and hypertension and its association with fasting insulin and central and overall obesity in a general population. Metabolism 1996;45:699ŌĆō706PMID : 8637443.


21. Moriwaki Y, Yamamoto T, Takahashi S, Tsutsumi Z, Higashino K. Apolipoprotein E phenotypes in patients with gout: relation with hypertriglyceridemia. Ann Rheum Dis 1995;54:351ŌĆō354PMID : 7794039.



22. Collantes Estevez E, Pineda Priego M, Anon Barbudo J, Sanchez Guijo P. Hyperuricemia-hyperlipidemia association in the absence of obesity and alcohol abuse. Clin Rheumatol 1990;9:28ŌĆō31PMID : 2335049.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



