INTRODUCTION
Recent advances in new devices and skills have broadened the indications of percutaneous coronary intervention (PCI). However, complications such as dissection, perforation, side branch jail and distal embolization of plaque materials and thrombi remain problems that can result in catastrophic outcomes1-3). There may be thrombi and plaque materials present related to the acute coronary syndrome, which have a high risk for embolization of neighboring arterial branches as well as distal small artery branches. The artery supplying the sinus node (SN) is very small and difficult to protect from jailing or plaque embolization during PCI. Acute occlusion of the SN artery can occur during PCI of proximal lesions of the right coronary artery (RCA) or the left circumflex artery (LCX)4). Therefore understanding of the prognosis of ischemic sinus node dysfunction is important for deciding on the need for permanent pacemaker implantation. We describe here the first reported case of transient sinus node dysfunction as a complication of balloon angioplasty in the distal LCX.
CASE REPORT
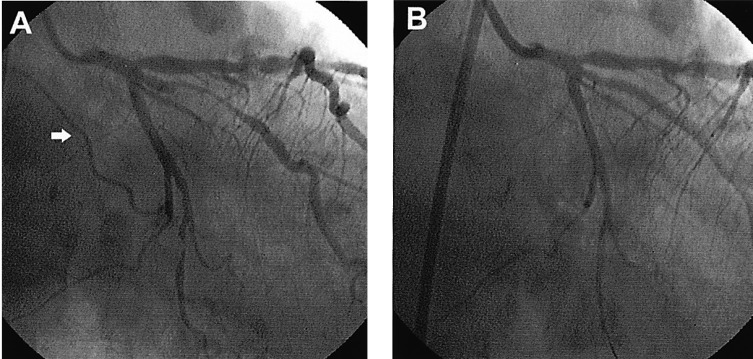
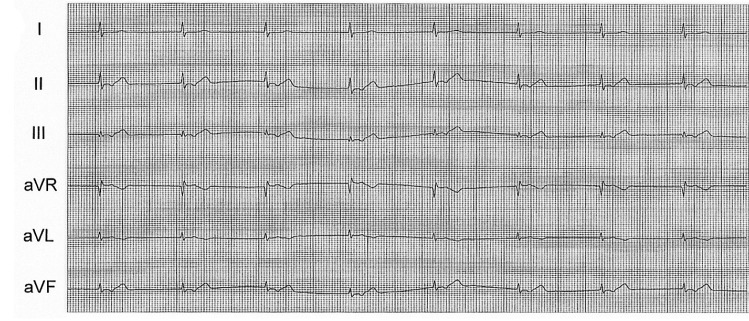
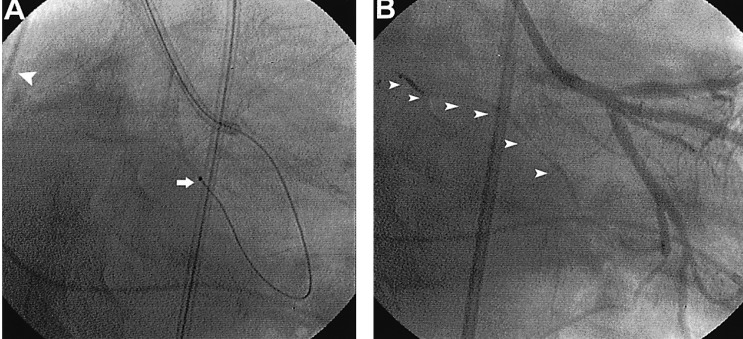
A 56-year-old male was admitted to the hospital with resting chest pain that developed three days before admission. He had effort-induced chest pain for three years, but did not take any medications and had no prior cardiac evaluations. Physical examination findings, blood pressure and heart rate were all normal. An echocardiography showed normal left ventricular wall motion and a normal ejection fraction. The electrocardiogram showed T wave inversions in lead aVL. The coronary angiography revealed a 50% discrete stenosis at the ostium of the left anterior descending artery (LAD), an 80% tubular stenosis at the intermedius branch, and a 90% tubular stenosis at the distal LCX (Figure 1A). A ramus intermedius angioplasty was successfully performed with placement of a sirolimus-eluting stent (Cypher, Johnson & Johnson). We performed a balloon dilatation with a balloon, 2.25 mm in diameter (Aqua, Johnson & Johnson). A coronary angiography immediately after balloon removal from the LCX demonstrated a patent distal LCX but total occlusion of the sinus node branch (Figure 1B). About 1 minute later, the patient complained of dyspnea and chest pain and the ECG showed a junctional rhythm with a heart rate of 40/min and the blood pressure dropped to 90/60 mmHg (Figure 2). A temporary pacemaker was inserted through the right subclavian vein and dopamine was continuously infused (10 ┬Ąg/kg/min) to increase the heart rate and blood pressure. The patient also received nitroglycerin (200 ┬Ąg) and abciximab (0.25 mg/kg) bolus through a guiding catheter; the abciximab was infused intravenously (10 ┬Ąg/min) for 12 hours. Then, an infusion catheter (Microferret, Cook) was placed in the SN artery where nitroglycerin 100 ug and nicorandil 1 mg were infused to rule out coronary spasm. Distal flow was not established; then urokinase 100,000 IU for 5 minutes through the catheter (Figure 3A) was infused. A coronary angiogram performed shortly after the urokinase infusion showed thrombolysis in acute myocardial infarction (TIMI).
DISCUSSION
The SN receives its major blood supply from the right coronary artery in 59% of cases, from the left coronary artery in 38% and from both coronary arteries in 3%5, 6). However, the incidence of duplication of the SN artery varies (1.4-11%)5, 7, 8). Detailed knowledge of the anatomy of the SN artery is beneficial for surgical procedures and angioplasty so that proper precautions can be taken to preserve the blood supply to the SN during PCI.
Actual occlusion of the sinus node artery, as occurred in our case, is not common. Procedures that may affect thrombi or vulnerable plaque around the SN artery must be performed carefully due to the possible mobilization of plaque or thrombi. There are several points to consider for prevention of mobilization of plaque or thrombus in the lesions associated with the acute coronary syndrome. First, removal of the balloon must be done only after complete deflation; this is because if incompletely deflated the bulky balloon may remove some of the plaque or thrombi that could embolize to the proximal side branches. In our case, we were not concerned about embolization because the SN artery was far from the lesion being repaired. However, an incompletely deflated balloon might have moved thrombus or plaque material from the lesion to the proximal SN artery. Second, deflation of the balloon should be done slowly. Rapid deflation of the balloon may cause movement of mural thrombi or plaque materials into the vessel lumen. Careful angioplasty, especially with an unstable lesion, is the most important measure for preventing mobilization or distal embolization of the thrombi or plaque.
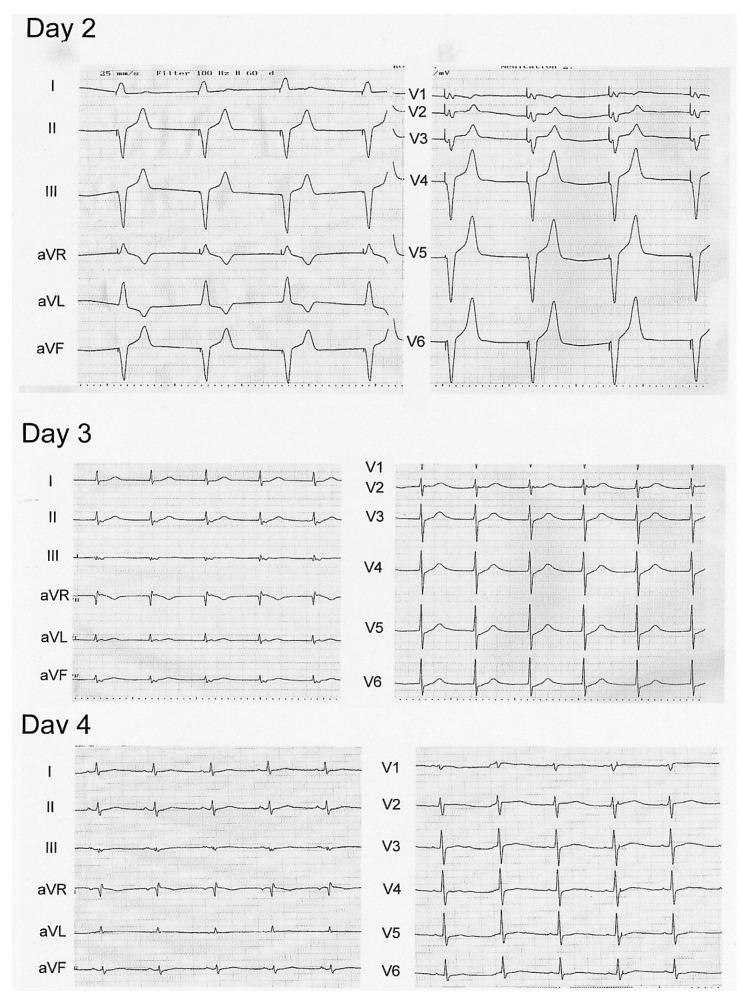
The SN is a mass of pace-making cells with higher metabolic requirements than other cells surrounding the atrial myocardium9). The SN has an abundant arterial supply and is generally resistant to ischemia10). The prognosis of acute ischemic injury to the SN is not completely understood in human patients. Chronic SN ischemia caused by a coronary artery anomaly or occlusion of the coronary artery can lead to a compromised blood supply to the sinus node, and hence cause the sick sinus syndrome11, 12). There are few reports on the prognosis in patients with acute ischemic SN dysfunction secondary to SN artery occlusion during PCI. Previously a report of one case of SN occlusion, following thrombosis of the SN artery during stent implantation, normalized spontaneously after one week in a patient with acute myocardial infarction.4 Prolonged SN ischemia can lead to exercise intolerance in young patients12, 13). In our patient, we suggest that thrombi in the LCX lesion might have embolized to the SN artery during balloon removal. The SN artery was successfully recanalized by local infusion with urokinase. However, we are not sure if this was helpful in the recovery of SN function. Although SN function in our patient worsened for two days after the procedure, the function was nearly completely recovered after seven days. More importantly, the patient an active young man did not complain of any symptoms. We have treated five other acute, transient flow disturbances of the SN artery and nodal dysfunction complicated by balloon angioplasty or stent implantation. In all of these cases the sinus rhythm recovered within an hour without any further interventions.
In conclusion, acute ischemic SN dysfunction may occur as a result of compromise of the SN artery during PCI. In addition, after establishing coronary flow, the decision to implant a permanent pacemaker should be delayed for one week after the insult to allow for spontaneous recovery.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





