INTRODUCTION
Cisplatin is one of the most effective anti-neoplastic agents used for treatment of various malignancies. However, 25-35% of patients who receive cisplatin experience nephrotoxicity to a varying degree [1]. Cisplatin causes tubular injury through multiple mechanisms, including hypoxia, oxidative stress, inflammation, and apoptosis. Furthermore, significant interactions among these various pathways may occur in cisplatin injury [2-6].
Recently, erythropoietin (EPO) was found to reduce injury caused by ischemiareperfusion in the brain, liver, heart, and kidney [7-10], and injury caused by cisplatin and cyclosporine [11,12]. EPO has anti-apoptic and antiinflammatory properties that protect tissues and enhance the regeneration of tubular epithelial.
Darbepoetin alfa (DPO), an erythropoietic agent with a serum half-life three times as long as EPO, exhibits similar protective effects [13] as well as hematopoietic effects, and attenuates renal ischemia-reperfusion injury in rats [14].
However, this compound has not yet been studied in cisplatin nephrotoxicity models in vivo. Thus, we examined whether DPO could attenuate cisplatin nephrotoxicity. We also examined the role of anti-apoptotic and antiinflammatory mechanisms in attenuating renal injury by DPO.
METHODS
Animals and drug treatment
All experiments were performed on 8-week-old male Sprague-Dawley (SD) rats (weighing 250-260 g; Korea Research Institute of Bioscience and Biotechnology, Daejeon, Korea). The rats were given a standard laboratory diet and water. Rats were cared for under a protocol approved by the Institutional Animal Care and Use Committee of the Chungnam National University Medical School.
We divided the rats into four groups: untreated (n=7), DPO-treated (n=7), cisplatin- injected (n=12), and DPO-treated cisplatin-injected (n=12) groups. The four groups were administered a single intraperitoneal injection of vehicle or cisplatin (5 mg/kg, Choongwae Pharma Co, Seoul, Korea). For the vehicle, 0.9% NaCl was administered in the same manner and volume. DPO treatment was performed with Darbepoetin (25 µg/kg once; Aranesp®, Amgen, Thousand Oaks, CA, USA), administered intraperitoneally 24 hours before cisplatin injection and just before cisplatin injection. The animals were sacrificed under anesthesia, by intraperitoneal administration of a mixture of ketamine (50 mg/mL, 10 mg/kg, Ketalar; Bayer, Leverkusen, Germany) and xylazine (2%, 0.1 mL/kg, Rompun; Bayer) in solution (2 mL/kg).
Tissue preparation
At the end of the study (96 hours after cisplatin injection), rats were anesthetized as described above. Both kidneys were excised immediately, and cut into three coronal sections. Two pieces of kidney were snap-frozen in liquid nitrogen, and stored at -70℃ for subsequent RNA extraction and protein analysis; the other piece was fixed in 10% buffered formaldehyde at room temperature, and then embedded in Paraplast (Sherwood Medical, St. Louis, MO, USA) for light microscopy and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL).
Light microscopy examination
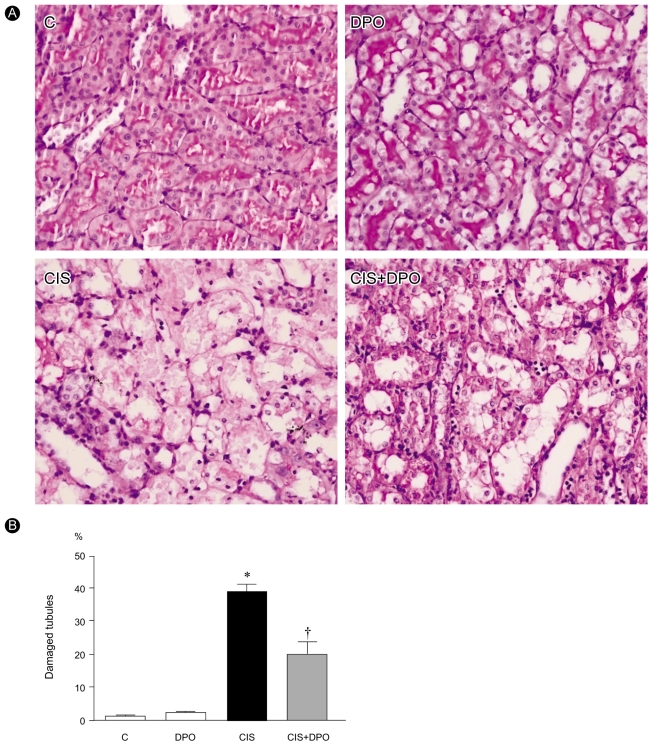
Pieces of kidney embedded in Paraplast were cut into 4-µm sections and mounted on glass slides. The sections were then deparaffinized with xylene, counterstained with hematoxylin and eosin (H&E), and examined under a light microscope (200× magnification, Dialux 22; Leitz, Milan, Italy). We evaluated necrosis, vacuolation, and desquamation of epithelial cells in renal tubules 96 h after cisplatin treatment. Five different fields of the renal outer-medular area of every slide were examined by a renal pathologist blind to the study groups. The magnitude of damage of tubular epithelial cells was examined in periodic acid Schiff (PAS)-stained sections, counting the numbers of tubules that were injured greater than 50% in the outer medulla.
RNA extraction, semi-quantitative RT-PCR and real-time PCR
Kidney sections stored at -70℃ were used for RNA extraction. Total RNA was extracted using the RNeasy Mini Kit (QIAGEN, Hilden, Germany). cDNA was synthesized from 1 µg total RNA, using an oligo dT primer (Amersham Pharmacia, Piscataway, NJ, USA), dNTPs (Amersham Pharmacia), M-MLV reverse transcriptase (Gibco-BRL, Grand Island, NY, USA), 0.1 M DTT, and buffers, in a volume of 20 µL. The 20-µL cDNA reaction mix was diluted to a total volume of 100 µL. Polymerase chain reaction (PCR) was then used to amplify the following specific cDNAs: GAPDH (primers: sense 5'TCC CAG ACC CCA TAA CAA CAG-3' and antisense 5'-TGA GGG TGC AGC GAA CTT TA3'), TNF-α (primers: sense 5' GTT GCC TCC CCC TTT TCT TT-3' and antisense 5'-CCT GGT CAC CAA ATC AGC ATT-3'), Fas (primers: sense 5'-CTG TCC TGC CTC TGG TGC TTG3' and antisense 5'-ACG AAC GCT CCT CTT CAA CTC C-3'), Bcl-2 (primers: sense 5'ACG GTG GTG GAG GAA CTC TTC-3' and antisense 5'ACA CAT GAC CCC ACC GAA CT3'), and MCP1 (primers: sense 5'-ATG CAG GTC TCT GTC ACG-3' and antisense 5'-ATG GTT CTC TGT CAT ACT-3'). PCR was performed using SYBR Green PCR mastermix (Applied by Qiagen). The amplification reaction volume was 20 µL, consisting of 10 µL iQ SYBR Green PCR mastermix, 1 µL primers, 1 µL cDNA, and 8 µL water. Amplification and detection were performed using a thermal cycler (Rotor-Gene 6000, Corbett Research, Mortlake, Australia). PCR conditions were as follows: denaturation at 95℃ for 10 minutes, followed by 40 cycles of 10 seconds at 95℃, 15 seconds at annealing temperature (60℃ for GAPDH, TNF-α, Fas, Bcl-2, MCP-1), and 20 seconds at 72℃. SYBR green fluorescence was measured at the end of each cycle using the comparative threshold cycle (Ct) method. The cDNA was quantified using the following formula for each group of data:
2-ΔΔCt = 2-[(Ct of target gene - Ct of GAPDH in DPO only-treated rat, IR rat, and DPO-treated cisplatin rat) - (Ct of target gene - Ct of GAPDH in untreated rat)].
Western blotting
Kidney sections stored at 70℃ were homogenized in Protein Extraction solution (PRO-PREP, iNtRON, Sungnam-si, Korea). Homogenates were centrifuged (12,000×g, 15 minutes, 4℃) and protein concentrations were determined in supernatants using the Bradford method protein microassay (BioRad, Hercules, CA, USA). The cleared supernatants were mixed with the same volume of 2× SDS sample buffer (62.5 mM, Tris-HCl (pH 6.8), 6% (w/v) SDS, 30% glycerol, 125 mM DTT, 0.3% (w/v) bromophenol blue), so that the final concentration of the mixture was 1× (500 µg/100 µL). The homogenates were boiled at 94℃ for 5 minutes in Laemmli sample buffer. Then, 40 µg total protein was loaded in a stacking polyacrylamide gel and resolved on an 8%+15% polyacrylamide gel with biotinylated molecular weight standard markers. The samples were then wet-transferred to a 0.2-micron nitrocellulose membrane (Amersham Pharmacia). The nitrocellulose membrane was stained with Coomassie blue to assess the equal loading of protein. Subsequently, the blots were blocked for 1 hour with 5% non-fat dry milk in TBST buffer (20 mM TrisHCl (pH 7.6), 0.8% NaCl, 0.05% Tween 20) and incubated overnight at 4℃ with a cleaved-caspase 3 antibody (1:1000 dilution; Cell Signaling Technology, Beverly, MA, USA), and a β-actin antibody (1:1000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA). After washing in TBST, the blots were incubated with secondary anti-rabbit IgG-HRP-linked antibody (1:1000 dilution; Cell Signaling Technology) for 1 hour. The blots were washed again in TBST and the bands were detected using enhanced chemiluminescence (Millipore, Billerica, MA, USA), and exposed to films. The optical density for quantification was determined using Gel-Pro Analyzer version 3.1 (Media Cybernetics, Silver Spring, MD, USA).
TUNEL
TUNEL was performed using the in situ Apoptosis Detection Kit (S7100-KIT; Chemicon). The procedure was modified from the manufacturer's protocol. Briefly, 4-µm-thick paraffin-embedded sections were dewaxed in three changes of xylene for 10 minutes each. Rehydration was achieved through a graded series of alcohols, from absolute to 95%, 80%, and 70% alcohol to water. After rinsing in PBS for 5 minutes, the sections were incubated in 0.3% H2O2 at room temperature to eliminate endogenous peroxidase activity. The sections were then washed in two changes of distilled water for 5 minutes each, followed by PBS for another 5 minutes. Proteinase K (10 µg/mL in 0.1 M Tris, 50 mM EDTA, pH 8) was applied to the sections, and incubated for 15 minutes at room temperature. After washing in four changes of distilled water for 2 minutes each, and one change of PBS for 5 minutes, two drops of equilibration buffer were applied to the sections for 0.5 to 1 minutes to facilitate pene-tration of terminal dUTP transferase, the TdT enzyme. A reaction mixture was prepared by mixing the reaction buffer and TdT enzyme at 5:1. Then this reaction mixture was applied to the sections, which were incubated in a humidified chamber for 1 hour at 37℃ to allow extension of the nick ends of the DNA fragments with digoxigenin-dUTP. During the incubation process, coverslips were put on the sections to prevent them from drying out. After the incubation, the coverslips were removed carefully without disturbing the tissues, and the sections were washed in stop buffer (1 mL stock in the kit + 34 mL distilled water) for 30 minutes at 37℃. After washing in PBS for 5 minutes, two drops of peroxidase-conjugated anti-DIG antibody were applied to the sections for 30 minutes at room temperature. After rinsing in three changes of PBS, followed by one change in Tris buffer, color was developed using 0.05% DAB with 0.006% H2O2 as a substrate. The sections were then washed in water, counterstained with methyl green, dehydrated through a graded alcohol series, cleared in xylene, and finally mounted with Permount for observation under a light microscope. For negative controls, distilled water was used instead of the TdT enzyme.
Statistical analyses
Data are reported as means±standard deviations (SD). Multiple comparisons between groups were performed using one-way analysis of variance with a post-hoc Bonferroni test correction (SPSS 11.0 for Windows; SPSS Inc., Chicago, IL, USA). Differences between groups were deemed to be statistically significant at p<0.05.
RESULTS
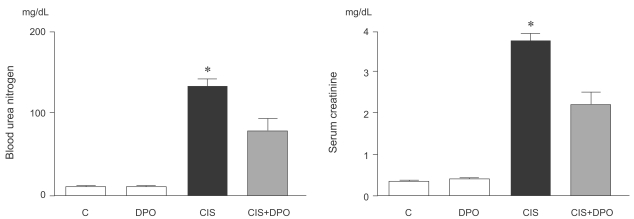
Effect of DPO on renal function in cisplatin nephrotoxicity
Cisplatin injection significantly increased blood urea nitrogen (BUN) and serum creatinine levels 96 h after injection. In contrast, intraperitoneal injection of DPO in cisplatin-injected rats significantly reduced BUN and serum creatinine levels, compared to rats treated with cisplatin alone (BUN, 143±25 mg/dL and creatinine, 3.9±0.2 mg/dL vs. BUN, 87.5±22.7 mg/dL and creatinine, 2.2±0.3 mg/dL, p<0.01, Fig. 1).
Effect of DPO on renal histology in cisplatin nephrotoxicity
Histological examination revealed loss of brush border, vacuolation, and desquamation of epithelial cells in renal tubular epithelium 96 h after injection in cisplatin-only treated rats. In contrast, treatment with DPO significantly improved cisplatin nephrotoxicity histologically (Fig. 2).
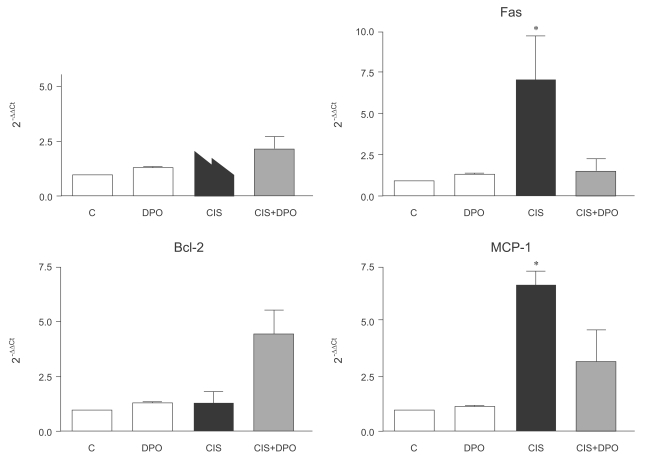
Anti-inflammatory and anti-apoptotic effects of DPO in cisplatin nephrotoxicity
In the reverse transcription RT-PCR analysis of inflammation- and apoptosis-related genes, expression of TNF-α increased significantly in kidneys of cisplatin-injected rats, compared to those of untreated and DPO-only treated rats (5.1±0.9 vs. 1.2±0.1, p<0.01). There were no differences in TNF-α mRNA expression between untreated and DPO-only treated rats. Treatment with DPO reversed the increased TNF-α mRNA expression in kidneys of cisplatin-injected rats (5.1±0.9 vs. 2.4±1.1, p<0.05). The expression of Fas increased significantly in kidneys of cisplatin-injected rats, compared to those of untreated and DPO-only treated rats (7.0±2.3 vs. 1.2±0.2, p<0.01). Treatment with DPO reversed the increased Fas mRNA expression in kidneys of cisplatin-injected rats (7.0±2.3 vs. 1.4±0.6, p<0.05). DPO treatment significantly increased Bcl-2 mRNA expression in kidneys of cisplatin-injected rats (1.3±0.4 vs. 4.3±1.6 OD, p<0.05). MCP-1 mRNA expression was significantly higher in the cisplatin-injected rats than in untreated and DPO-only treated rats (6.6±1.2 vs. 1.2±0.1, p<0.01), but was significantly down-regulated in DPO-treated cisplatin-injected rats (6.6±1.2 vs. 2.7±1.5, p<0.05). No difference in MCP-1 mRNA expression was detected in the kidneys of untreated and DPO-only treated rats (Fig. 3).
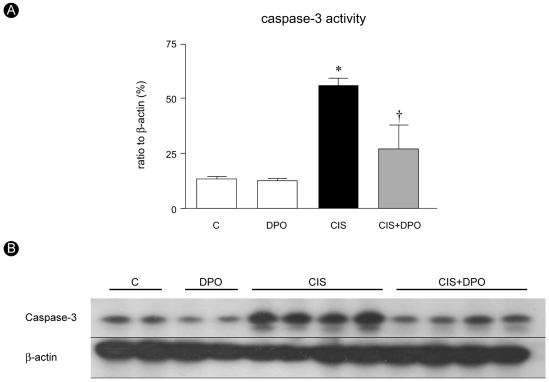
Immunoblotting showed that caspase-3 activity increase significantly in cisplatin-injected kidneys compared to untreated and DPO only treated kidneys (53.2±8.2% vs. 14.6±1.9%, OD, p<0.01). DPO administration significantly lowered the elevated caspase-3 activity in cisplatin-injected rats (53.2±8.2% vs. 27.0±8.8%, OD, p<0.01), while no difference in caspase-3 activity was detected between untreated and DPO-only treated rats (Fig. 4). DPO treatment significantly decreased the number of TUNEL positive cells in cisplatin-treated rats (70.8±30.8 vs. 21.5±8.3, p<0.01, Fig. 5).
DISCUSSION
We demonstrated that DPO has a renoprotective effect on cisplatin nephrotoxicity in rat kidneys, by attenuating inflammation and apoptosis of renal tubular epithelial cells. The pathogenesis of renal injury by cisplatin is associated with oxidative stress, inflammation, and apoptosis [2]. Apoptosis is an important mode of cell death in cisplatin nephrotoxicity. Engagement of a cell surface receptor with extracellular TNF-α activates caspase 8 [15]. Activated caspases 1, 8, and 9 are initiator caspases that activate caspase 3, the principal executioner caspase in renal tubule apoptosis. Additionally, while cisplatin can induce Fas clustering into the membrane lipid rafts and induce an apoptosis cascade in the absence of Fas ligand in cancer cells, whether it is involved in nephrotoxicity is unknown [16]. Inflammation also helps mediate cisplatin-induced renal injury. Ramesh and Reeves [17] showed that TNF-α may play an important role in inflammation and apoptosis in renal tubular epithelial cells in cisplatin nephrotoxicity models. They also demonstrated that a TNF-α-neutralizing antibody had protective effects on a cisplatin nephrotoxicity model [18]. As potent inflammatory markers, MCP-1 and TGF-β are also increased in the kidney by cisplatin [19].
DPO is a hyperglycosylated analog of recombinant human erythropoietin that exhibits the same mechanism of action as erythropoietin but a three-fold longer terminal half-life after intravenous administration in both animal models and humans [20]. It is as effective as EPO, with a similar safety profile and less frequent dosing, in anemia associated with chronic renal failure [21]. If it were demonstrated to exhibit similar effects as those observed with EPO, this would suggest that it has a pharmacokinetic profile that would be clinically advantageous in ARF, acute renal failure.
EPO has protective effects on acute kidney injury. Both low-dose (300 U/kg) and high-dose EPO (5000 U/kg) have renal protective effects in ischemiareperfusion injury [14,22,23]. DPO has a tissue-protective effect at low (2 µg/kg) and high (30 µg/kg) doses in cardiac ischemia-reperfusion models [24]. Both delayed treatment and pretreatment of DPO (25 ug/kg) have renoprotective effects on renal ischemia-reperfusion injury [14].
EPO's anti-apoptotic effects are mediated via activation of JAK-2 and subsequent nuclear translocation of NF-κB and transcription of anti-apoptotic genes, including expression of Bcl-XL and X-linked inhibitor of apoptosis. An additional protective mechanism is an anti-apoptotic effect via activation of PI3K/Akt and of STAT-family transcriptional factor. The anti-inflammatory action of EPO leads to the suppression of TNF-α and MCP-1 in cerebral ischemia and autoimmune encephalomyelitis models [25,26].
Although DPO has effects that are similar to EPO, the renal protective mechanism(s) of DPO remain unclear. DPO ameliorates apoptosis in porcine tubular and murine mesangial cells induced by toxic and hypoxic stimuli [27]. The anti-apoptotic effect of DPO may be associated with Bax protein suppression and prolonged Akt activation [14]. In a cisplatin nephrotoxicity model, Vaziri et al. [28] reported that EPO may act directly by stimulating tubular cell regeneration.
In the present study, DPO treatment suppressed caspase-3 protein levels and TUNEL-positive cells in the kidneys of cisplatin nephrotoxicity rats. This may suggest that DPO exerts anti-apoptotic effects. The potential protective mechanisms of DPO include decreased expression of pro-apoptotic TNF-α, and increased expression of anti-apoptotic Bcl-2, as assessed using real-time RT-PCR. These results suggest that the renoprotective effect of DPO in cisplatin nephrotoxicity occurs at least partly by reducing apoptosis. These results are consistent with previous observations of anti-apoptotic effects of DPO in ischemic ARF in vivo [14] and in mouse mesangial cells subjected to toxic stimuli [27]. DPO suppressed the expression of MCP-1 in kidneys of rats with cisplatin nephrotoxicity. This suggests that DPO treatment also suppressed the inflammatory process caused by cisplatin.
We found significant increases in hematocrit over the 5day study period in animals receiving DPO. Although a hemopoietic response may have a potentially beneficial effect by increasing oxygencarrying capacity, it may also harm kidneys by enhancing thrombotic potential [29]. However, previous in vitro studies are consistent with the beneficial effects of EPO in acute kidney injury being independent of its hemopoietic actions [10,27]. Additionally, asialoerythropoietin, which is generated by the enzymatic desialylation of EPO, provides neuroprotection without changing the hematocrit. Thus, there is evidence for tissue-protective effects of EPO independent of hematocrit.
In conclusion, our study demonstrates that inflammation and apoptosis may be involved in the mechanism of kidney injury in cisplatin nephrotoxicity. We showed that DPO has a renoprotective effect against inflammation and apoptosis in the kidneys of cisplatin-treated rats.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





