INTRODUCTION
Upper gastrointestinal (GI) bleeding is a common medical emergency in clinical practice, and Dieulafoy lesions are an important cause of potentially life-threatening GI bleeding. Typically, these lesions consist of large-caliber submucosal arteries in close contact with the mucosa over a variable distance, and massive bleeding can occur with erosion of the mucosa and arterial wall [1]. These lesions are responsible for 0.5 to 14% of acute upper GI bleeding [2]. Most lesions are located in the proximal stomach, but they can also be found in other GI tract locations [3]. The therapy for this condition has evolved from surgery to endoscopy.
In addition to the different endoscopic techniques used for GI bleeding, such as injection with or without thermal therapy, mechanical methods such as the use of hemoclips and rubber band ligation have recently been used.
The aims of the study were to assess the efficacy of endoscopic treatment for Dieulafoy lesions and to identify possible predictive factors for rebleeding in patients with these lesions.
METHODS
Patients
Between January 2006 and December 2007, 312 patients with nonvariceal upper GI bleeding were admitted to Pusan National University Hospital. A total of 44 patients with a Dieulafoy lesion in the upper GI tract were examined. After basic life support was provided, all patients underwent emergent endoscopy. All procedures were performed by two expert endoscopists within 12 hours of patient admission. Informed consent was obtained from every patient or from family members. All patients received only topical analgesics and an intravenous proton pump inhibitor. No systemic sedative agent was given to any patient. Follow-up endoscopy was performed within 24 hours of the initial procedure and at 7 days. Patient data were collected during hospitalization and included demographic information, medical history, initial hemodynamic status, laboratory values, endoscopic findings, endoscopic therapy, and outcome (i.e., complications, death, rebleeding, and the need for surgery). Information on rebleeding and mortality following discharge was obtained during follow-up outpatient visits. Because this study was a retrospective review, institutional review board approval was not necessary.
Definitions
The endoscopic diagnosis of a Dieulafoy lesion was based on the following established criteria [3]: 1) active arterial spurting or micropulsatile streaming from minute (<3 mm) mucosal defects, 2) visualization of a protruding vessel with or without active bleeding within a minute mucosal defect with normal surrounding mucosa, or 3) a densely adherent clot with a narrow attachment point to a minute mucosal defect or normal-appearing mucosa. Initial hemostatic failure was defined as sustained active bleeding despite initial endoscopic management or any evidence of active bleeding, such as hematemesis, hematochezia, or hemodynamic instability (systolic blood pressure <100 mm Hg, pulse rate >100 beats/minutes, or an orthostatic change in systolic blood pressure >20 mm Hg or a pulse rate >20 beats/minutes) within 12 hours of the initial hemostasis. Rebleeding was suspected in patients with additional episodes of hematemesis or in those with melena, hemodynamic instability, or a decrease in hemoglobin concentration of at least 2 g/dL in 24 hours and diagnosed when the endoscopy showed bleeding from a previously treated lesion.
Statistical analysis
The Mann-Whitney test was used to compare the mean values of continuous variables, and Fisher's exact test was used to compare non-ratio variables. The analysis was conducted with SPSS version 13.0 (SPSS Inc., Chicago, IL, USA). A p value of less than 0.05 was accepted as statistically significant.
RESULTS
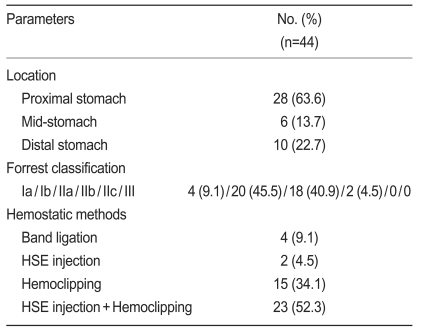
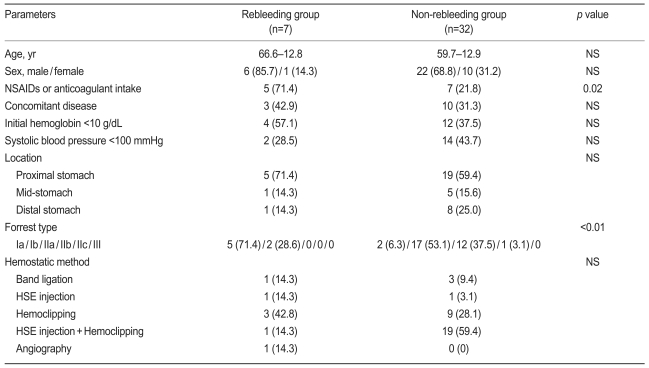
During the study period, 44 Dieulafoy lesions were identified among 312 endoscopies performed for nonvariceal upper GI bleeding (Table 1). The locations of the bleeding lesions were as follows: proximal stomach, 28; mid-stomach, 6; and distal stomach, 10. In all, 24 patients (54.5%) were bleeding at the time of diagnosis: 4 had spurting, and 20 had an oozing hemorrhage. The remaining 20 patients had a nonbleeding visible vessel or an adherent clot. The following hemostatic methods were used as therapeutic procedures: band ligation (4 patients), hypertonic saline-epinephrine injection (2 patients), hemoclipping (15 patients), and hypertonic saline-epinephrine injection with hemoclipping (23 patients). In the treatment group, primary hemostasis was achieved in 39 patients (88.6%). Five patients with initial failure of hemostasis required emergent transarterial embolization because of continuous bleeding at sites difficult to approach. In three, permanent hemostasis was achieved by transarterial embolization. In addition, two patients received surgery. Seven patients (17.9%) had recurrent bleeding 1-3 days after the initial endoscopic procedure, and another endoscopic hemoclipping or angiography with embolization resulted in permanent hemostasis. There were no significant differences between the rebleeding and non-rebleeding groups with respect to age, gender, initial hemoglobin levels, presence of shock, concurrent disease, bleeding location, or initial hemostatic methods. However, there was a statistically significant difference in the use of non-steroidal anti-inflammatory drugs (NSAIDs) or anticoagulants and in the Forrest classification of bleeding (p=0.02 and p<0.01, Table 2). A summary of the management and treatment outcomes is provided in Fig. 1. Among the 44 patients discharged from the hospital, 6 were lost to follow-up and 38 were available for assessment. The mean follow-up was 15 months (range, 5 to 29). During the outpatient follow-up, there was no recurrence of bleeding, and there were no procedure-related complications.
DISCUSSION
Although Dieulafoy lesions are an uncommon cause of GI bleeding [4], the results of our study show them to be a relatively frequent cause, accounting for 14.1% of nonvariceal upper GI bleeding. As in previous reports, many patients had significant comorbidities, and the ingestion of aspirin, NSAIDs, or warfarin was common. The clinical features of the patients with Dieulafoy lesions were similar to those of patients reported previously [2,5-8].
Endoscopic therapy, which has emerged as the mainstay for managing Dieulafoy lesions, is safe and highly successful in terms of achieving initial hemostasis [2,9]. There are many reports of successful hemostasis using a variety of endoscopic modalities, including injection of sclerosants [2,10,11], thermal coagulation [5,10], and mechanical methods such as band ligation [12] or hemoclip application [13]. The success rate of various forms of endoscopic therapy range from 75 to 98% [10,14-16]. In the present study, primary hemostasis was achieved in 39 patients (88.6%). If endoscopic therapy fails, management with other options such as transarterial embolization or surgery is indicated. Five patients with initial failure of hemostasis required emergent transarterial embolization and surgery, and all had permanent hemo-stasis. Seven patients (17.9%) had rebleeding 1-3 days after the initial endoscopic procedure, and endoscopic retreatment and transarterial embolization resulted in permanent hemostasis.
There was a significant difference in the intake of NSAIDs and anticoagulants as well as in the type of bleeding between the rebleeding group and the non-rebleeding group.
Long-term results for patients treated by endoscopic methods are excellent [12,17,18], and mortality is generally low with prompt diagnosis and treatment. In our study, the mean follow-up was 15 months. During the outpatient follow-up, there was no recurrence of bleeding, and there were no procedure-related complications.
This study has several limitations. The first limitation is the small sample size and the retrospective design. Medical records that are not designed for research purposes may not include all of the variables of interest or may contain inaccurate descriptions. In addition, there was limited power to show statistical differences because of the small sample size. The second limitation is that this series of patients was enrolled from a single institution and reflected a number of factors associated with this institution; therefore, the post-discharge outcome data may be incomplete. However, we believe that most patients discharged from our hospital would return to the same institution if upper GI bleeding recurred. The third limitation is the impact of treatment. Although we limited the drug options for acute bleeding, the drugs chosen reflect the preferences of the clinical physicians; however, we believe these drug options did not influence the results of the study.
Despite these limitations, this study remains important for demonstrating the efficacy of endoscopic treatment for Dieulafoy lesions and the factors associated with rebleeding in the context of these patients and the diagnostic and treatment algorithm.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





