 |
 |
| Korean J Intern Med > Volume 25(3); 2010 > Article |
|
Abstract
Marginal zone B-cell lymphoma (MZL) is the second most common subtype of non-Hodgkin's lymphoma in Korea (17.3%). Mucosa-associated lymphoid tissue (MALT) can develop in almost any organ as a result of exposure to a persistent stimulus, such as chronic infection or certain autoimmune processes. Under conditions of prolonged lymphoid proliferation, a malignant clone may emerge, which is followed by the development of a MALT lymphoma. Whereas MALT lymphoma of the stomach is the most common and the most extensively studied, we focus on non-gastric MZL studies conducted in Korea that highlight the most recent advances with respect to MZL definition, etiology, clinical characteristics, natural history, treatment approaches, outcomes, and prognostic factors. Moreover, we discuss current organ-specific considerations and controversies, and identify areas for future research.
Marginal zone B-cell lymphoma (MZL) is a type of lymphoma in which the cells originate from B lymphocytes that are normally located in the "marginal zone" of the secondary lymphoid follicles. Depending on the site of involvement, three distinct subgroups are defined by the WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues [1].
Extranodal MZL of mucosa-associated lymphoid tissue (MALT lymphoma) is an extranodal lymphoma composed of morphologically heterogeneous small B cells, including marginal zone (centrocyte-like) cells, monocyte-like cells, small lymphocytes, and scattered immunoblasts and centroblast-like cells. Plasma cell differentiation occurs in the majority of MALT lymphomas. The infiltrate occurs in the marginal zone of reactive B-cell follicles and extends into the interfollicular region. In the epithelial tissues, the neoplastic cells typically infiltrate the epithelium, forming lymphoepithelial lesions.
Splenic MZL is a B-cell neoplasm composed of small lymphocytes, which surround and replace the spleen white pulp germinal centers, efface the follicle mantle, and merge with a peripheral (marginal) zone of larger cells, including scattered transformed blasts; both small and larger cells infiltrate the red pulp. Splenic hilar lymph nodes and the bone marrow (BM) are frequently involved; lymphoma cells may be detected in the peripheral blood as villous lymphocytes.
MZL represents a distinct subgroup of non-Hodgkin's lymphoma (NHL), which is typically characterized by an indolent clinical course and long survival time [2-4]. In Korea, MZL comprises 17.3% of NHLs, while MALT lymphomas account for 16.7% and nodal MZL accounts for 0.6% of all NHLs and 23% of B-cell lymphomas [5]. MZL is the second most frequent histologic subtype after diffuse large B-cell lymphoma (DLBCL). Every year, an estimated 500 patients are newly diagnosed with MZL [6]. However, the International Lymphoma Study Group has reported that MZL comprises 8% of NHL cases. Nodal and splenic MZL account for 16.0% and 7.6% of MZL cases, respectively [7]. Significant variability in the incidence of MZL has been reported for different geographic regions.
Risk factors for extranodal MZLs have been identified. These malignancies are associated with the acquisition of MALT in organs that normally lack organized lymphoid tissues, e.g., the stomach, salivary glands, thyroid, conjunctiva, skin, and other organs. The acquisition of MALT is induced by autoimmune disease or chronic inflammation.
The presence of Helicobacter pylori (H. pylori) [8], Borrelia burgdorferi [9], Chlamydia psittaci (Cp) [10], and Campylobacter jejuni [11] may be related to stomach, skin, ocular, and intestinal MZL, respectively. Other extranodal MZLs have been associated with a continuous immune-triggering mechanism involving autoantigens. Higher incidences of lymphomas of the salivary and lachrymal glands, thyroid, and lung have been noted for patients with Sj├Čgren's syndrome, Hashimoto's thyroiditis, and lymphoid interstitial pneumopathy, respectively [12,13]. In two Korean studies on non-gastric MZL (NG-MZL), Cp DNA was detected in 60% and 78%, respectively, of patients with ocular MZL [14,15]. Associations between other infectious organisms and MZL remain unclear, owing to a lack of relevant data.
At a single center in Korea, gastric MZL accounted for 50% of all patients with MZL [16,17]. In patients with NG-MZL, the most commonly involved sites are (in decreasing order of frequency) the orbit and ocular adnexa (48.9%), lymph node and lymphatic organs (17.8%), bowel (9.3%), lungs (6.1%), thyroid (4.9%), and salivary glands (4.5%) [18]. The organ distribution of NG-MZL varies with the geographic region. In a European patient survey, the salivary gland (25%), ocular and adnexa (25%), lungs (14%), and skin (12%) were identified as the main sites of NG-MZL [2].
In a large cohort of Korean patients with NG-MZL [18], the overall male-to-female ratio was almost 1:1. Even though autoimmune diseases (e.g., Hashimoto's thyroiditis and Sj├Čgren's syndrome) occur more commonly in females, the percentages of patients with thyroid and salivary gland MZL were lower than those presented in the international data set; therefore, the gender ratio was not significantly affected [2,3]. The median age of the cohort was 49 years. The median patient age was approximately 10 years younger. In Korea, orbital and ocular adnexa MZL (OA-MZL) accounts for approximately 50% of NG-MZL cases, and OA-MZL tends to be detected early, owing to its anatomical location [18].
In these patients, B symptoms, poor performance status, and large mass size of the lymphoma are quite rare, being observed in < 5% of patients. Increased levels of lactic dehydrogenase (LDH) are observed in approximately 10% of patients, and 75% of the patients initially present with localized disease (defined by Ann Arbor stage I/II). BM involvement is seen in < 10% of the patients. Approximately 90% of the patients were categorized into the low or low-intermediate risk groups according to the International Prognostic Index (IPI), while 80% of the patients were categorized as being in the low-risk group according to the Follicular Lymphoma International Prognostic Index (FLIPI).
In Korean MZL analysis, the principal clinical features were limited stage, small tumor burden, excellent performance status, and normal LDH. However, patients with extranodal MZL and those with nodal MZL show differences in clinical presentation. Patients with nodal MZL are distinguished by male predominance, higher incidence of B symptoms, BM involvement, poor performance status, advanced stage, and assignment to the high-risk group according to IPI and FLIPI [18,19].
The optimal clinical treatment for MZL remains to be clearly defined. Antibiotic therapy, surgery, radiation, immunotherapy, and chemotherapy, applied either singly or in combination, have been previously employed in clinical practice, including watchful wait.
Localized stage MZL can be controlled with local modalities, such as radiotherapy and/or surgery. In retrospective study [18], of the 151 patients with stage I or II disease, 138 (91.4%) were treated with local modalities, such as radiation therapy or surgery, with or without systemic chemotherapy. The overall response (OR) rate was 98%, with 140 patients showing complete responses (CRs) and 8 patients having partial responses (PRs). Notably, the CR rate for patients who received radiotherapy was 96% (108/113 patients), whereas only 66.7% (8/12) of the patients who received primary chemotherapy achieved a CR. Locoregional recurrence (75%) was more common than distant site recurrence in the patients with limited-stage MZL, regardless of the treatment modality [18,20]. The 5-year progression-free survival (PFS) and overall survival (OS) rates were 74.7% and 95.9%, respectively. No significant differences in survival were observed between the patients with nodal or extranodal MALT involvement.
Based on the clinical data obtained for the patients with MZL in this retrospective study, local treatment/radiotherapy should be considered the principal treatment modality.
Given the indolent natural history of MZL, less-toxic chemotherapeutic or immunotherapeutic agents are preferable. In this respect, considering whether anthracyclines should be used in the treatment of advanced-stage MZL is important. To date, only one retrospective study has addressed this issue [18]. Of the 36 patients with advanced-stage MZL who were treated with chemotherapy, 20 received a chemotherapy regimen that contained an anthracycline. The OR rate in that study was 75%, with 17 patients having a CR and 10 patients showing a PR. Although the CR rate was significantly higher in patients who were treated with the anthracycline-based chemotherapy than in those treated with a non-anthracycline-based regimen (65% vs. 25%, p = 0.023), the PFS (p = 0.556) and OS (p = 0.554) for these two groups were similar. Therefore, the advantage of using anthracycline in the treatment of advanced-stage MZL appears to be in improving the response rate rather than having a significant impact on survival.
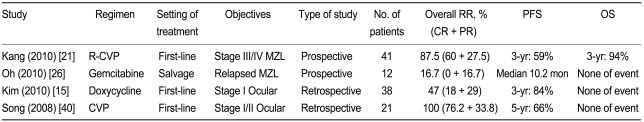
Another major issue in MZL treatment is whether rituximab confers a survival benefit in patients with advanced disease. Based on the results of retrospective analyses, a prospective clinical trial of rituximabcyclophosphamide, vincristine, and prednisolone (R-CVP) as a first-line treatment for advanced-stage MZL was conducted [21]. Between March 2006 and July 2008, 42 patients were enrolled in this trial at 13 institutes in Korea. The patients received a total of 287 cycles of R-CVP chemotherapy (median, 8 cycles/person; range, 3 to 8 cycles/person). Twenty-four patients achieved CR (60%), 11 had PR (27.5%), 4 had stable disease (10%), and 1 had progressive disease (2.5%), yielding an OR rate of 87.5% (95% confidence interval [CI], 77.1 to 97.9). Toxicity-related hospitalization was required for 9 patients (22.5%) during treatment. However, no treatment-related deaths occurred. After a median follow-up of 30.7 months (range, 11.1 to 43.8), the estimated 3-year PFS and OS rates were 59.5% and 95.0%, respectively. Although defining the value of rituximab is difficult in a single-arm phase II trial, the R-CVP regimen appears to be effective and well tolerated in patients with advanced-stage MZL (Table 1).
In patients with MZL having long survival times, relapses are common. Overall, more than 50% of these patients experience a relapse within 10 years [2,18,22-25]. Relapsed or refractory MZL is treated according to the disease stage and disease location.
In patients with stage I or II MZL, limited-stage locoregional recurrence (up to 75%) was more commonly observed than distant-site relapse, regardless of the radiotherapy or chemotherapy regimen used [18,20]. Many of these patients achieved a CR (54.1%) or a PR (18.9%) with radiotherapy or chemotherapy. In particular, radiotherapy resulted in a high rate of CRs (68.8%).
Almost all of the patients who had advanced-stage disease at relapse were treated with chemotherapy. More than half of the patients achieved either a CR (40.9%) or PR (13.6%). The median PFS was 34.1 months (95% CI, 11.3 to 56.9 months), and the estimated 5-year OS was 84.3%. The relevant predictive clinical factors for reduced PFS and OS were refractory MZL and advanced relapse MZL.
Patients with relapsed MZL tend to experience frequent relapses and prolonged survival. Although the second PFS period is generally shorter than the first PFS period, the overall survival time is consistently long.
On the basis of retrospective analyses [20], salvage gemcitabine monotherapy was used to treat relapsed/refractory advanced-stage MZL [26]. However, this trial was halted early because the response rate (16.7%) did not reach the initial assumed minimal response rate at stage I (Table 1).
Since MZL cases tend to exhibit recurrent relapse with long survival times, further clinical trials should be conducted to develop a more effective and safer treatment modality.
The IPI and FLIPI have been used as prognostic indices for NHL and indolent lymphoma. However, MZL has a distinctive clinical presentation and a natural course. IPI has been used for predicting NHL prognosis [27]. However, IPI was originally designed for aggressive lymphoma, and for low- and intermediate-risk cases, the discriminating power of IPI is somewhat limited [28,29]. For indolent lymphoma, most patients are categorized in the low- and intermediate-risk groups. Thus, FLIPI has been employed for indolent lymphoma, as is the case for follicular lymphoma (FL) [30]. Although MZL and FL exhibit the same indolent nature, they are regarded as distinct disease entities. In a minority of patients with MZL, the involvement of more than four nodes has been noted, and no definitive evidence exists concerning the effects of low hemoglobin values on PFS and OS rates in cases of MZL [2,31].
In a retrospective multivariate analysis of the Korean data, nodal MZL and advanced stage were predictive of the PFS in a multivariate analysis. With regard to OS, poor performance status and advanced stage were introduced as predictive factors in the multivariate analysis [18,32].
Given the indolent nature of MZL, OS may be influenced more by comorbidity than by MZL itself. Thus, the performance status of patients may constitute an important factor for OS. However, the PFS was influenced by a variety of factors associated with disease status, rather than by patient comorbidities.
A low percentage of MZLs (< 5% in 5 years) transform into large-cell, aggressive lymphomas, which are predictive of poor prognosis for MZL OS [32].
Of the MALT sites of NG-MZL, the OA-MZLs have been studied the most extensively in terms of genetic changes, etiology, and treatment (with antibiotics, chemotherapy, or radiotherapy) in Korea. OA-MZLs present as slowly enlarging lesions that arise from the eyelid, orbit, lachrymal gland, or conjunctiva. OA-MZLs account for approximately 50% of all NG-MZL cases and more than 90% of ocular lymphoma cases [33-35]. Compared to the data sets from other countries, the Korean studies exhibit higher rates of conjunctival involvement (up to 50% of OA-MZLs).
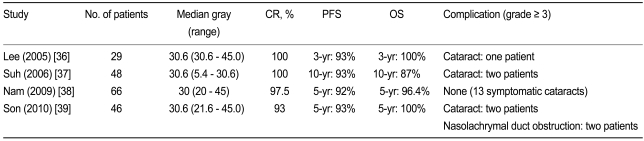
Localized OA-MZL can be controlled effectively using radiotherapy, as evidenced by the four retrospective Korean studies conducted to date [36-39] (Table 2). Although the data were analyzed retrospectively, excellent responses were achieved with a relatively low dosage of radiation (median, 30.6 Gy). The CR rates ranged from 93% to 100%, and the 5-year PFS and OS rates were > 90% and > 95%, respectively. These results demonstrate that localized OA-MZL can be controlled effectively with low-dosage radiation, and that the benefits of radiotherapy persist for a long time (> 5 years).
Despite its effective local control of tumors, radiotherapy can have ophthalmologic complications, skin irritation or mild conjunctivitis, lens toxicity, corneal complications, xerophthalmia, retinal complications, and cataracts. However, in the Korean study, severe complications, particularly cataracts (grade Ōēź 3), were reported in < 5% of the patients. Therefore, low-dosage radiotherapy administered alone at a median level of 30.6 Gy, in conjunction with appropriate lens shielding, may prove to be the optimal therapeutic modality for localized OA-MZL [38,39].
A correlation between Cp infection and OA-MZL has been suggested in several previous studies. Ferreri et al. [10] demonstrated an association between OA-MZL and infection with Cp in Italian patients. In that study, Cp-DNA was detected by a combination of immunohistochemistry (using a monoclonal antibody against Cp lipopolysaccharide) and PCR analysis in 80% of 40 lymphoma samples. Similar findings were reported in Korea, whereby Cp-DNA was detected in 79% of patients [14]. However, Cp-positivity has been shown to be variable in meta-analyses of geographic regions (overall, 25%; range, 0 to 88%).
Moreover, the use of doxycycline for the eradication of H. pylori from patients with gastric MZL remains controversial. Only one Korean retrospective study has been published to date [15]. In this trial, 38 patients with newly diagnosed, localized OA-MZL received doxycycline for 3 weeks (n = 12) or 6 weeks (n = 26). After a median follow-up of 26.4 months, doxycycline treatment yielded an OR rate of 47% and a 3-year time-to-treatment failure (TTF) rate of 84%. In a multivariate analysis, absolute lymphocytosis (odds ratio, 4.7; 95% CI, 1.1 to 20.8; p = 0.043) and non-conjunctival tumor (odds ratio, 11.8; 95% CI, 1.1 to 122.5; p = 0.038) were identified as negative predictors of response. Front-line doxycycline is particularly effective in patients with localized OA-MZL with conjunctival involvement but without absolute lymphocytosis. Cp-DNA was noted in 15/25 (60%) patients for whom tumor tissues were available. However, Cp-positivity was not associated with the doxycycline response (60% vs. 60%, p = 1.000). Considering the efficacy of doxycycline and the indolent nature of MZL, antibiotic therapy to eradicate Cp may represent an alternative treatment for elderly patients with OA-MZL or a tool for use in clinical trials (Table 1).
Despite exerting effect local control of tumors, radiotherapy has ophthalmologic toxic side effects, which included moderate cutaneous or conjunctiva reactions, and late complications, such as constant cataract, xerophthalmia, rare ischemic retinopathy, glaucoma, and corneal ulceration. In addition to these side effects, distant site relapse is associated with chemotherapy of localized OA-MZL.
In a retrospective analysis [40], CVP combination chemotherapy resulted in an OR rate of 100% (CR, 76.2%). After a median follow-up of 58 months, 14/21 (66.7%) patients were free of disease, while 7 patients showed disease progression, manifested as extra-orbital (n = 2) or local failures (n = 5). Radiotherapy was delivered to five patients with local failure, and these patients subsequently achieved a CR with late ophthalmologic complications. Tolerable adverse events were associated with the CVP regimen. Based on these findings, first-line CVP combination chemotherapy, in conjunction with radiotherapy in recurrent cases, appears to be effective and well tolerated in patients with localized OA-MZL. To confirm the efficacy of this first-line chemotherapy in conjunction with radiotherapy for patients with recurrent disease, further prospective clinical trials are needed (Table 1).
Primary NHL of the lung is a very rare condition, accounting for only 0.4% of all malignant lymphomas [41]. MALT lymphoma is the most common subtype of pulmonary NHL [42]. Smoking and chronic inflammatory conditions of the lung have been identified as etiologic agents of P-MZL [43,44]. In the Korean retrospective data set, 63.2% of the patients had a history of smoking, which is consistent with most reports on this type of patient cohort. Another notable finding in the Korean study was that 11.5% of the students had a history of tuberculosis [45].
With regard to clinical features, approximately 50% of the patients initially presented without any symptoms. Lobar or segmental consolidation was the most frequently observed feature, being detected in 68.9% of the patients in this study. However, since P-MZL exhibits a diversity of lung lesion patterns on computed tomography scans [46] (i.e., nodular, consolidation, and with diffuse infiltrative patterns), estimating diagnostically and prognostically significant values was difficult. Based on the previous series, common bronchofiberoscopy may be of limited diagnostic value [43,47,48]. In the Korean retrospective data [45], video-assisted thoracic surgery (VATS) and direct open lobectomy were conducted for approximately 50% of the patients. However, the remaining 50% of the patients were diagnosed using less-invasive procedures, such as percutaneous needle aspiration and cytology (PCNA), and bronchofiberoscopic biopsy without surgery. This suggests that less-invasive diagnostic procedures should be considered prior to surgery in these patients.
The optimal management of P-MZL lymphoma in terms of surgery, chemotherapy, and radiation therapy alone or in combination, as well as abstention from therapy, remains to be established. From the perspective of MZL, localized P-MZL, which is limited to one side of the lung, may be a marker of a favorable response to local radiation or surgery [43,44,49]. In addition, advanced or disseminated P-MZL, which involves bilateral lungs or extrapulmonary sites, could be controlled by chemotherapy. However, in the lung, even though the lesions are localized, radiation and surgical excision of segments or lobes should be considered carefully given the risk of surgical complications, reductions in organ function, and the favorable clinical course of MZL itself. In the Korean data set [45], 56 of the 61 patients were treated with surgery (n = 22), chemotherapy (n = 28), or radiotherapy (n = 6). Of these, 46 patients (82.1%) achieved complete or partial remission. The median PFS was 5.6 (95% CI, 2.6 to 8.6) years. We detected no differences between chemotherapy and surgery in terms of PFS (p = 0.617). This was the case for patients with either single-lobe or unilateral P-MZL. Therefore, in those patients for whom surgery is not required for diagnosis, to preserve lung function and avoid the risks associated with surgery, surgery may not be the first-choice treatment for P-MZL.
From the results of the Korean survey, P-MZL tends to be an indolent disease, being characterized by long-term survival with frequent relapses, similar to other MALT-type site MZLs. To preserve lung function and limit the risks associated with surgery, chemotherapy should be regarded as a first-line option for the treatment of P-MZL.
In a previous report on gastrointestinal (GI) NHL, I-MZL accounted for 2.7% of all GI NHL cases [50]. In cases of NG-MZL, I-MZL was noted in 1% to 5% of all cases of extragastric MZL. The clinical presentations were similar to those of other NG-MZL sites. I-MZL commonly appears as an early-stage, low-risk state [2,51].
In the Korean data set [52], the small intestine was identified as the second most common site of GI NHL involvement, after the stomach. The most frequently observed I-MZL involvement site was the ileocecal region (40.7%), and rectal MZL (15%) was observed more frequently than MZL of the colon (4%). The most common complaints made by patients with I-MZL were abdominal pain (62.9%), diarrhea (22.2%), and bleeding (15%). Advanced-stage disease was observed at a higher frequency for I-MZL than for MZL at other sites. Musshoff's stages IE, IIE1, IIE2, IIIE, and IV were identified in 44%, 15%, 11%, 7.4%, and 22% of cases, respectively.
Approximately 71% of the patients presented with Musshoff's stage I-II. Considering the clinical features of MZLs, local treatment can be regarded as the principal treatment modality. A high CR rate was achieved with various treatments, including local modalities. Even for patients with advanced-stage disease, 62.5% underwent surgery, since almost all suffered from subjective symptoms and the small intestine and ileocecal region are difficult regions in which to conduct endoscopic biopsy tissue diagnoses. CR and PR were achieved in 82% and 4% of the patients, respectively. The estimated 5-year OS and PFS rates were 86% and 54%, respectively. Stage Ōēź IIE2 was identified as a poor prognostic factor for PFS and OS.
Regardless of stage, I-MZL can be controlled relatively well with local or systemic treatments, as is the case with MZL at other sites.
Nodal MZL (N-MZL) is a relatively uncommon variant of lymphoma. In previous reports, B-cell NHL incidences of < 1% have been reported. In a recent report, N-MZL was identified as a distinct disease entity rather than an advanced stage of MALT-type MZL. The clinical presentations and survival outcomes differ for these two types of MZL [31,53,54].
N-MZL has an incidence of < 1% in Korea [5]. The clinical presentation of N-MZL is slightly different from that of MALT lymphoma. In a Korean retrospective analysis [19], 53% of the patients had localized disease (stages I and II), and 21.2% (7/33) had BM involvement at presentation. B symptoms were present in only three patients (8.3%). Most of the patients were categorized as low or low-intermediate risk using the IPI (77.1%). A relatively high proportion of cases of N-MZL presented with advanced stage, poor performance status, and high IPI and FLIPI scores, i.e., more like FL than MALT lymphoma.
Localized N-MZL was successfully treated using either local treatment modalities (radiotherapy, surgical resection, or chemotherapy) or systemic chemotherapy with or without involved field radiotherapy, as in MALT lymphoma. A majority (94.4%) of the patients with localized disease achieved a CR after the initial treatment.
The superiority of an anthracycline-containing regimen for the treatment of patients with advanced N-MZL remains a matter of controversy. Patients with disseminated N-MZL who had primary anthracycline-based chemotherapy were likely to achieve a CR and showed a favorable PFS. However, no definite prolongation of OS was observed according to the type of chemotherapy regimen administered. The median PFS was 3.9 (95% CI, 2.9 to 5.6) years, and the estimated 5-year PFS and OS rates were 47.2% and 82.7%, respectively.
In the Korean lymphoma incidence studies, S-MZL accounted for < 1% of all MZL cases [5,18]. Why S-MZL occurs so rarely is difficult to explain. Although splenectomy is required for the diagnosis of S-MZL, up to two-thirds of patients with circulating S-MZL present with circulating villous lymphocytes having characteristic fine cytoplasmic polar projections [55]. Almost all patients with primary S-MZL are older than 60 years [56,57]. S-MZL presents with massive splenomegaly coupled with abdominal discomfort. Peripheral LN enlargement, except for splenic hilar LN, is absent. Nearly all patients show evidence of infiltrated BM [56,57].
The clinical course of S-MZL is generally indolent, with 5-year OS rates that range from 65% to 80% [56-58]. When treatment is required, it is usually because of symptomatic splenomegaly or cytopenia. The preferred treatment, splenectomy, results in a reduction or disappearance of circulating tumor lymphocytes and recovery of the lymphoma-associated cytopenia. The benefits conferred by splenectomy often persist for several years, and the time to the next treatment can be more than 5 years in patients in which lymphocytosis persists and/or progresses after splenectomy.
Chemotherapy alone may be considered for patients who require treatment but in whom splenectomy is contraindicated, and also for patients who show clinical progression after splenectomy. Alkylating agents and fludarabine have been reported to be active, and can be used as single agents or in combination. Rituximab, alone or in combination with chemotherapy, has been reported to induce responses in patients who are refractory to standard chemotherapy [57,59,60]. Treatment of hepatitis C virus (HCV) infection with interferon-╬▒, alone or in combination with ribavirin, may be helpful for patients having splenic lymphoma with villous lymphocyte and HCV infection [61,62].
Studies on MZL in Korea may generate important benefits in terms of diagnosis and treatment. The relatively high incidence of MZL in Korea should provide sufficient numbers of patients for clinical trials, and should generate much informative data about the disease. In planning future clinical trials of MZL, at least two systems must first be established and implemented. The first of these is a well organized study group. Even though the incidence of MZL is higher in Korea than in Western countries, the absolute numbers of newly diagnosed patients are low. The establishment of a well organized nationwide study group would help to overcome this drawback. The second prerequisite is cooperation among clinical departments, such as those in pathology, radiation oncology, ophthalmology, and gastroenterology. All clinical trials must include a central pathology review. In addition, MZL entails organ-specific considerations, which are necessary for a multidisciplinary approach to patients with MZL.
Several key questions remain to be answered. Does the optimal first-line treatment for localized or advanced MZL include rituximab, chemotherapy, radiotherapy or watchful wait? Should patients with relapsed MZL be treated with less-toxic immunotherapy, salvage conventional chemotherapy, radioimmunotherapy, or high-dose chemotherapy? How we can predict the transformation of indolent MZL to aggressive DLBCL?
Regarding the Korean lymphoma study, if the past 5 years are collectively referred to as the "age of retrospective data collection," the near future will be referred to as the "age of prospective clinical trials and translational studies."
References
1. Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Pathology and Genetics of Tumours of Haematolopoietic and Lymphoid Tisses. 2008;Lyon: IARC Press.
2. Zucca E, Conconi A, Pedrinis E, et al. Nongastric marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue. Blood 2003;101:2489ŌĆō2495PMID : 12456507.


3. Zinzani PL, Magagnoli M, Galieni P, et al. Nongastrointestinal low-grade mucosa-associated lymphoid tissue lymphoma: analysis of 75 patients. J Clin Oncol 1999;17:1254. PMID : 10561186.


4. Thieblemont C, Berger F, Dumontet C, et al. Mucosa-associated lymphoid tissue lymphoma is a disseminated disease in one third of 158 patients analyzed. Blood 2000;95:802ŌĆō806PMID : 10648389.


5. Ko YH, Kim CW, Park CS, et al. REAL classification of malignant lymphomas in the Republic of Korea: incidence of recently recognized entities and changes in clinicopathologic features. Hematolymphoreticular Study Group of the Korean Society of Pathologists. Revised European-American lymphoma. Cancer 1998;83:806ŌĆō812PMID : 9708949.


6. Won YJ, Sung J, Jung KW, et al. Nationwide cancer incidence in Korea, 2003-2005. Cancer Res Treat 2009;41:122ŌĆō131PMID : 19809561.




7. The Non-Hodgkin's Lymphoma Classification Project. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma. Blood 1997;89:3909ŌĆō3918PMID : 9166827.



8. Wotherspoon AC. Gastric MALT lymphoma and Helicobacter pylori. Yale J Biol Med 1996;69:61ŌĆō68PMID : 9041690.


9. Roggero E, Zucca E, Mainetti C, et al. Eradication of Borrelia burgdorferi infection in primary marginal zone B-cell lymphoma of the skin. Hum Pathol 2000;31:263ŌĆō268PMID : 10685647.


10. Ferreri AJ, Guidoboni M, Ponzoni M, et al. Evidence for an association between Chlamydia psittaci and ocular adnexal lymphomas. J Natl Cancer Inst 2004;96:586ŌĆō594PMID : 15100336.


11. Lecuit M, Abachin E, Martin A, et al. Immunoproliferative small intestinal disease associated with Campylobacter jejuni. N Engl J Med 2004;350:239ŌĆō248PMID : 14724303.


12. Hyjek E, Isaacson PG. Primary B cell lymphoma of the thyroid and its relationship to Hashimoto's thyroiditis. Hum Pathol 1988;19:1315ŌĆō1326PMID : 3141260.


13. Isaacson PG. Extranodal lymphomas: the MALT concept. Verh Dtsch Ges Pathol 1992;76:14ŌĆō23PMID : 1283245.


14. Yoo C, Ryu MH, Huh J, et al. Chlamydia psittaci infection and clinicopathologic analysis of ocular adnexal lymphomas in Korea. Am J Hematol 2007;82:821ŌĆō823PMID : 17570512.


15. Kim TM, Kim KH, Lee MJ, et al. First-line therapy with doxycycline in ocular adnexal mucosa-associated lymphoid tissue lymphoma: a retrospective analysis of clinical predictors. Cancer Sci 2010;101:1199ŌĆō1203PMID : 20345477.


16. Kim JH, Kim WS, Ko YH, et al. Clinical investigation of gastric MALT lymphoma. Korean J Med 2001;61:417ŌĆō423.
17. Oh SY, Kim WS, Kim JH, et al. Extra-gastric MALT lymphoma: Analysis of 50 cases. Korean J Med 2000;59:261ŌĆō267.
18. Oh SY, Ryoo BY, Kim WS, et al. Nongastric marginal zone B-cell lymphoma: analysis of 247 cases. Am J Hematol 2007;82:446ŌĆō452PMID : 17266060.


19. Oh SY, Ryoo BY, Kim WS, et al. Nodal marginal zone B-cell lymphoma: analysis of 36 cases. Clinical presentation and treatment outcomes of nodal marginal zone B-cell lymphoma. Ann Hematol 2006;85:781ŌĆō786PMID : 16847665.


20. Oh SY, Kim WS, Kim SJ, et al. Relapsed or refractory nongastric marginal zone B-cell lymphoma: multicenter retrospective analysis of 92 cases. Am J Hematol 2009;84:826ŌĆō829PMID : 19890833.



21. Kang HJ, Ryoo BY, Suh C, et al. Rituximab plus CVP (cyclophosphamide, vincristine, and prednisolone) combination chemotherapy on advanced stage marginal zone B-cell lymphoma. Haematologica 2010;95(s2):623.
22. Suh C, Huh J, Roh JL. Extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue arising in the extracranial head and neck region: a high rate of dissemination and disease recurrence. Oral Oncol 2008;44:949ŌĆō955PMID : 18234544.


23. Isaacson PG. Update on MALT lymphomas. Best Pract Res Clin Haematol 2005;18:57ŌĆō68PMID : 15694184.


24. Zucca E, Bertoni F, Stathis A, Cavalli F. Marginal zone lymphomas. Hematol Oncol Clin North Am 2008;22:883ŌĆō901PMID : 18954742.


25. Ferreri AJ, Zucca E. Marginal-zone lymphoma. Crit Rev Oncol Hematol 2007;63:245ŌĆō256PMID : 17583528.


26. Oh SY, Kim WS, Lee DH, et al. Phase II study of gemcitabine for treatment of patients with advanced stage marginal zone B-cell lymphoma: Consortium for Improving Survival of Lymphoma (CISL) trial. Invest New Drugs 2010;28:171ŌĆō177PMID : 19421710.


27. The International Non-Hodgkin's Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med 1993;329:987ŌĆō994PMID : 8141877.


28. L├│pez-Guillermo A, Montserrat E, Bosch F, Terol MJ, Campo E, Rozman C. Applicability of the International Index for aggressive lymphomas to patients with low-grade lymphoma. J Clin Oncol 1994;12:1343ŌĆō1348PMID : 8021724.


29. Foussard C, Desablens B, Sensebe L, et al. The GOELAMS Group, France. Is the International Prognostic Index for aggressive lymphomas useful for low-grade lymphoma patients? Applicability to stage III-IV patients. Ann Oncol 1997;8(Suppl 1):49ŌĆō52PMID : 9187429.

30. Solal-C├®ligny P, Roy P, Colombat P, et al. Follicular lymphoma international prognostic index. Blood 2004;104:1258ŌĆō1265PMID : 15126323.


31. Nathwani BN, Anderson JR, Armitage JO, et al. Marginal zone B-cell lymphoma: a clinical comparison of nodal and mucosaassociated lymphoid tissue types. Non-Hodgkin's Lymphoma Classification Project. J Clin Oncol 1999;17:2486ŌĆō2492PMID : 10561313.


32. Oh SY, Kwon HC, Kim WS, et al. Nongastric marginal zone B-cell lymphoma: a prognostic model from a retrospective multicenter study. Cancer Lett 2007;258:90ŌĆō97PMID : 17936499.


33. Oh DE, Kim YD. Lymphoproliferative diseases of the ocular adnexa in Korea. Arch Ophthalmol 2007;125:1668ŌĆō1673PMID : 18071120.


34. Cho EY, Han JJ, Ree HJ, et al. Clinicopathologic analysis of ocular adnexal lymphomas: extranodal marginal zone b-cell lymphoma constitutes the vast majority of ocular lymphomas among Koreans and affects younger patients. Am J Hematol 2003;73:87ŌĆō96PMID : 12749009.


35. Yoon JS, Ma KT, Kim SJ, Kook K, Lee SY. Prognosis for patients in a Korean population with ocular adnexal lymphoproliferative lesions. Ophthal Plast Reconstr Surg 2007;23:94ŌĆō99.


36. Lee JL, Kim MK, Lee KH, et al. Extranodal marginal zone B-cell lymphomas of mucosa-associated lymphoid tissue-type of the orbit and ocular adnexa. Ann Hematol 2005;84:13ŌĆō18PMID : 15309523.


37. Suh CO, Shim SJ, Lee SW, Yang WI, Lee SY, Hahn JS. Orbital marginal zone B-cell lymphoma of MALT: radiotherapy results and clinical behavior. Int J Radiat Oncol Biol Phys 2006;65:228ŌĆō233PMID : 16503386.


38. Nam H, Ahn YC, Kim YD, Ko Y, Kim WS. Prognostic significance of anatomic subsites: results of radiation therapy for 66 patients with localized orbital marginal zone B cell lymphoma. Radiother Oncol 2009;90:236ŌĆō241PMID : 18950885.


39. Son SH, Choi BO, Kim GW, et al. Primary radiation therapy in patients with localized orbital marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT Lymphoma). Int J Radiat Oncol Biol Phys 2010;77:86ŌĆō91PMID : 19632068.


40. Song EK, Kim SY, Kim TM, et al. Efficacy of chemotherapy as a first-line treatment in ocular adnexal extranodal marginal zone B-cell lymphoma. Ann Oncol 2008;19:242ŌĆō246PMID : 17947227.


41. Ferraro P, Trastek VF, Adlakha H, Deschamps C, Allen MS, Pairolero PC. Primary non-Hodgkin's lymphoma of the lung. Ann Thorac Surg 2000;69:993ŌĆō997PMID : 10800781.


42. Ahmed S, Siddiqui AK, Rai KR. Low-grade B-cell bronchial associated lymphoid tissue (BALT) lymphoma. Cancer Invest 2002;20:1059ŌĆō1068PMID : 12449739.


43. Ahmed S, Kussick SJ, Siddiqui AK, et al. Bronchial-associated lymphoid tissue lymphoma: a clinical study of a rare disease. Eur J Cancer 2004;40:1320ŌĆō1326PMID : 15177490.


44. Zinzani PL, Poletti V, Zompatori M, et al. Bronchus-associated lymphoid tissue lymphomas: an update of a rare extranodal maltoma. Clin Lymphoma Myeloma 2007;7:566ŌĆō572PMID : 18186964.


45. Oh SY, Kim WS, Kim JS, et al. Pulmonary marginal zone B-cell lymphoma of MALT type--what is a prognostic factor and which is the optimal treatment, operation, or chemotherapy: Consortium for Improving Survival of Lymphoma (CISL) study. Ann Hematol 2010;89:563ŌĆō568PMID : 20024551.


46. Bae YA, Lee KS, Han J, et al. Marginal zone B-cell lymphoma of bronchus-associated lymphoid tissue: imaging findings in 21 patients. Chest 2008;133:433ŌĆō440PMID : 18071012.


47. Xu HY, Jin T, Li RY, Ni YM, Zhou JY, Wen XH. Diagnosis and treatment of pulmonary mucosa-associated lymphoid tissue lymphoma. Chin Med J (Engl) 2007;120:648ŌĆō651PMID : 17517178.


48. Kim JH, Lee SH, Park J, et al. Primary pulmonary non-Hodgkin's lymphoma. Jpn J Clin Oncol 2004;34:510ŌĆō514PMID : 15466823.


49. Stefanovic A, Morgensztern D, Fong T, Lossos IS. Pulmonary marginal zone lymphoma: a single centre experience and review of the SEER database. Leuk Lymphoma 2008;49:1311ŌĆō1320PMID : 18604720.


50. Koch P, del Valle F, Berdel WE, et al. Primary gastrointestinal non-Hodgkin's lymphoma: I Anatomic and histologic distribution, clinical features, and survival data of 371 patients registered in the German Multicenter Study GIT NHL 01/92. J Clin Oncol 2001;19:3861ŌĆō3873PMID : 11559724.


51. Kohno S, Ohshima K, Yoneda S, Kodama T, Shirakusa T, Kikuchi M. Clinicopathological analysis of 143 primary malignant lymphomas in the small and large intestines based on the new WHO classification. Histopathology 2003;43:135ŌĆō143PMID : 12877728.


52. Oh SY, Kwon HC, Kim WS, et al. Intestinal marginal zone B-cell lymphoma of MALT type: clinical manifestation and outcome of a rare disease. Eur J Haematol 2007;79:287ŌĆō291PMID : 17692101.


53. Conconi A, Bertoni F, Pedrinis E, et al. Nodal marginal zone B-cell lymphomas may arise from different subsets of marginal zone B lymphocytes. Blood 2001;98:781ŌĆō786PMID : 11468179.


54. Nathwani BN, Drachenberg MR, Hernandez AM, Levine AM, Sheibani K. Nodal monocytoid B-cell lymphoma (nodal marginalzone B-cell lymphoma). Semin Hematol 1999;36:128ŌĆō138PMID : 10319381.

55. Troussard X, Valensi F, Duchayne E, et al. Splenic lymphoma with villous lymphocytes: clinical presentation, biology and prognostic factors in a series of 100 patients. Groupe Francais d'H├®matologie Cellulaire (GFHC). Br J Haematol 1996;93:731ŌĆō736PMID : 8652403.


56. Chac├│n JI, Mollejo M, Mu├▒oz E, et al. Splenic marginal zone lymphoma: clinical characteristics and prognostic factors in a series of 60 patients. Blood 2002;100:1648ŌĆō1654PMID : 12176884.


57. Thieblemont C, Felman P, Callet-Bauchu E, et al. Splenic marginal-zone lymphoma: a distinct clinical and pathological entity. Lancet Oncol 2003;4:95ŌĆō103PMID : 12573351.


58. Bertoni F, Zucca E. State-of-the-art therapeutics: marginal-zone lymphoma. J Clin Oncol 2005;23:6415ŌĆō6420PMID : 16155028.


59. Arcaini L, Orlandi E, Scotti M, et al. Combination of rituximab, cyclophosphamide, and vincristine induces complete hematologic remission of splenic marginal zone lymphoma. Clin Lymphoma 2004;4:250ŌĆō252PMID : 15072617.


60. Paydas S, Yavuz S, Disel U, Sahin B, Ergin M. Successful rituximab therapy for hemolytic anemia associated with relapsed splenic marginal zone lymphoma with leukemic phase. Leuk Lymphoma 2003;44:2165ŌĆō2166PMID : 14959867.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



