 |
 |
| Korean J Intern Med > Volume 25(3); 2010 > Article |
|
Abstract
Background/Aims
Many patients with aspirin-induced asthma have severe methacholine airway hyperresponsiveness (AHR), suggesting a relationship between aspirin and methacholine in airway response. This study was performed to determine whether methacholine AHR affects the response of asthmatics to inhaled aspirin.
Methods
The clinical records of 207 asthmatic patients who underwent inhalation challenges with both aspirin and methacholine were reviewed retrospectively. An oral aspirin challenge was performed in patients with a negative inhalation response. The bronchial reactivity index (BRindex) was calculated from the percent decrease in lung function divided by the last dose of the stimulus.
Results
Forty-one (20.9%) and 14 (7.1%) patients showed a positive response to aspirin following an inhalation and oral challenge, respectively. Only 24.3 and 14.3% of the responders had a history of aspirin intolerance, respectively. The methacholine BRindex was significantly higher in the inhalation responders (1.46 ± 0.02) than in the oral responders (1.36 ± 0.03, p < 0.01) and in non-responders (n = 141, 1.37 ± 0.01, p < 0.001). The aspirin BRindex was significantly correlated with the methacholine BRindex (r = 0.270, p < 0.001). Three of four patients who received the oral challenge, despite a positive inhalation test, showed negative responses to the oral challenge. Two of these patients had severe AHR.
Conclusions
A considerable number of asthmatic patients with no history of aspirin intolerance responded to the inhalation aspirin challenge. The airway response to aspirin was significantly correlated with methacholine-AHR, and a false-positive response to aspirin inhalation test seemed to occur primarily in patients with severe AHR.
Asthmatic symptoms in approximately 14 to 29% of adult asthmatics are exaggerated after the ingestion of aspirin [1]. Aspirin-induced asthma (AIA) usually develops as persistent rhinosinusitis with nasal polyposis, followed by asthma and aspirin hypersensitivity [2]. The syndrome is increasingly being referred to as aspirin-exacerbated respiratory disease, as exposure to aspirin does not initiate the disease, but does provoke strong asthmatic attacks in the later stages of the disease.
Although a patient's clinical history may give rise to suspicion of AIA, the diagnosis can only be established with certainty by aspirin provocation testing. A controlled oral challenge with aspirin is regarded as the gold standard for diagnosing AIA; however, the widely used three-day challenge proposed by Manning and Stevenson [3] and even the recently advocated two-day challenge protocol [4] are time consuming and can produce severe asthmatic reactions [5]. Consequently, an inhalation challenge with lysine-aspirin is the preferred method.
The inhalation aspirin challenge was first introduced by Bianco et al. [6] in 1977. It has since been demonstrated that the inhalation challenge is less sensitive than oral challenges, although it is safer and takes less time to perform [2,7]. The oral challenge is regarded as positive when severe extra-bronchial symptoms, such as nasal blockade, appear even if the forced expiratory volume in 1 second (FEV1) does not decrease > 20% [4]. An inhalation challenge rarely induces extra-bronchial symptoms. In addition, it is reasonable to speculate that results from an inhalation challenge are affected by the patient's underlying airway hyperresponsiveness (AHR). We have found that irritable airways constrict easily even in response to inhaled normal saline aerosols (unpublished data), and the bronchial response to an inhaled allergen is significantly related to methacholine AHR [8].
In previous sgudies [9-14] of 25 or fewer subjects, airway responses to inhaled aspirin did not correlate with AHR. Methacholine AHR was not significantly different between 23 patients with AIA and 23 aspirin-tolerant asthmatic subjects [10]. The degree of airway responsiveness to lysine-aspirin was not related to histamine AHR in 11 [11] and 9 [12] AIA patients, or to methacholine AHR in 10 [13] and 25 AIA patients [14]. As AIA does not occur simply as an epiphenomenon of severe asthma but develops in a small subset of patients with asthma who have a genetic predisposition [15], airway responsiveness to aspirin and to methacholine or histamine are unlikely to be closely related.
Approximately half of the patients with AIA are oral steroid-dependent, and thus, many of them have developed severe AHR [16,17]. Therefore, AIA is related to AHR in a broad sense, and a significant relationship could be confirmed if studied using large numbers of subjects. Moreover, inhalation rather than an oral challenge could induce bronchospasms more easily in irritable airways. It may also be possible for airways with severe AHR to respond non-specifically to aspirin. Nonetheless, there are no reports of a significant relationship between AIA and AHR or of the irritant effects of aspirin in spasmodic airways. Here, we report that airway responsiveness to inhaled aspirin is influenced by underlying AHR in asthmatic patients.
The clinical records of 207 asthmatic patients who underwent both inhalation aspirin and methacholine challenge testing at our hospital between 1997 and 2008 were reviewed retrospectively. Of these patients, 178 (86.0%) were adult men, 17 to 30 years old, seeking a medical certificate for exemption from obligatory military service. Thus, many of the subjects included in this study had no previous history of an adverse reaction to aspirin or other non-steroidal anti-inflammatory drugs. The patients underwent several tests including an aspirin challenge to diagnose asthma. The subjects were instructed to refrain from using any medication for at least 7 days before the tests. The study protocol was approved by the Institutional Review Board of our hospital (IRB No. I-2008-07-091).
Asthma was diagnosed if at least one of the following criteria was fulfilled: the provocative concentration of methacholine that resulted in a 20% fall in FEV1 (PC20) was ≤ 16 mg/mL; histamine-PC20 ≤ 16 mg/mL; a provocative dose of inhaled 4.5% hypertonic saline resulting in a 15% fall in FEV1 < 20 mL; a maximal % fall in FEV1 ≥ 15% to an exercise test; or a change in FEV1 ≥ 12% and ≥ 200 mL spontaneously or following treatment for asthma. Increased numbers of blood eosinophils and serum total IgE concentrations were defined as > 450/mm3 [18] and > 100 IU/mL, respectively. A positive serum level of house dust mite-specific IgE was defined as > 3.5 kU/L (≥ 3+) measured using UniCAP®100ε (Pharmacia Diagnostics, Uppsala, Sweden) for Dermatophagoides farinae and/or Dermatophagoides pteronyssinus. To diagnose aspirin intolerance, an aspirin challenge was performed according to the method proposed by Melillo et al. [13] with modifications. Briefly, the patients underwent an inhalation aspirin challenge and, if the test result was negative, received oral doses of 250 and 500 mg of aspirin at 2-hour intervals to exclude aspirin sensitivity not related to the inhalation challenge. The patients were classified into three groups based on their inhalation and oral aspirin challenge responses. Group I had a positive response to the inhalation test, Group II had a positive response to the oral challenge, and Group III had negative responses to both tests. Eleven patients showed a negative response to the inhalation test but did not receive the oral challenge and were excluded from the classification.
A methacholine bronchoprovocation test was performed on the first day, followed by administration of the aspirin challenge on the second day. The methacholine test followed a standardized tidal breathing protocol [19]. The FEV1 was measured in triplicate before testing and in duplicate at 30 and 90 s after each inhalation period using a spirometer (SpiroAnalyzer ST-250, Fukuda Sangyo, Tokyo, Japan). The highest FEV1 from the acceptable spirograms was selected as the representative value for each period [20]. The selected predictive equation for FEV1 was that recommended by the Intermountain Thoracic Society [21]. Isotonic saline followed by methacholine (Sigma-Aldrich, St. Louis, MO, USA) was aerosolized at room temperature in a DeVilbiss 646 nebulizer (output 0.13 mL/min; DeVilbiss, Somerset, PA, USA). The dilution increments were 0.075, 0.15, 0.31, 0.62, 1.25, 2.5, 5.0, 10, and 25 mg/mL. The aerosols were inhaled by tidal breathing over 2 minutes at 5-minute intervals through the mouth with the nose clipped. The challenge test was discontinued if the FEV1 dropped by 20% or more from the post-saline FEV1, or if the maximum concentration of methacholine was administered. The PC20 was calculated by linear interpolation of the log dose-response curve. The severity of methacholine AHR was categorized by a modification of the method of Woolcock and Jenkins [22] as follows: severe, PC20 < 0.2; moderate, PC20 = 0.2 to 2.0; mild, PC20 = 2.0 to 16.0; and normal, PC20 > 16 mg/mL. As actual PC20 values could not be obtained for subjects with a negative test response, the bronchial reactivity index (BRindex) as an index of AHR was calculated as follows: log10 (10 + maximal % fall in FEV1 / log10 [dose in mg/dL of methacholine required to produce the response]) [23].
An inhalation aspirin challenge was performed according to the method of Phillips et al. [11]. Patients inhaled increasing doses (11.25, 45, 180, and 360 mg/mL) of lysine-aspirin (Ilyang, Seoul, Korea; 360 mg of lysine-aspirin is equivalent to 200 mg of aspirin) after the saline challenge using a dosimeter (Microdosimer, SM Instruments Co. Ltd., Doyles Town, PA, USA). The aerosols were inhaled ten times from the end tidal volume to full inspiratory capacity at 30-minute intervals, and the FEV1 was measured at 10, 20, and 30 minutes after each inhalation period. The lowest FEV1 of the three measurements was selected as the representative value for each period [4]. The inhalation challenge was discontinued if the FEV1 dropped by 20% or more from the post-saline FEV1, or if the maximum concentration of lysine-aspirin was administered. The maximum cumulative dose of aspirin calculated according to European guidelines [4] was 29.8 mg. A dose-response curve was constructed, and the BRindex for aspirin was calculated using an equation similar to that shown above for methacholine. In subjects who ingested aspirin, the FEV1 was measured 1 hour after ingestion and extrapulmonary symptoms were recorded. A positive response to aspirin was defined as a decrease in FEV1 ≥ 20% from the post-saline value or a fall in FEV1 ≥ 15% with extrapulmonary symptoms of intolerance such as rhinorrhea and nasal congestion [24]. AIA was defined as a positive response to the inhalation or oral aspirin challenge.
All data were analyzed by the Kruskal-Wallis test, Mann-Whitney U test, χ2 test, Fisher's exact test, or Pearson's correlation using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). All data are shown as the mean ± standard error of the mean (SEM). A p < 0.05 was considered statistically significant.
The 196 asthmatic patients included in this study were classified into three groups based on their inhalation and oral aspirin challenge responses. Forty-one subjects (20.9%) had a positive response to the inhalation test (Group I), 14 subjects (7.1%) had a positive response to the oral challenge (Group II), and 141 subjects (71.9%) had negative responses to both tests (Group III). The remaining 11 patients, who were excluded from the classification, showed a negative response to the inhalation test but did not receive the oral challenge. Thus, a total of 55 (28.1%) patients were diagnosed as AIA; the proportion of false negative diagnosis following the inhalation challenge was 25.5%. The clinical characteristics of the subjects are summarized in Table 1. No differences in sex, age, height, duration of asthma, associated allergic rhinitis and sinusitis, blood eosinophilia, increased total IgE level, or positivity for house dust mite-specific IgE were observed among the groups. However, the prevalence rates of a positive history of aspirin intolerance were significantly different among the groups (24.3, 14.3, and 7.4% for Groups I, II, and III, respectively; p < 0.05). In five (35.7%) of the 14 patients in Group II, the oral challenge was considered positive on the basis of extra-bronchial symptoms even though the FEV1 decreased < 20% from the post-saline value. When only the data of men aged ≤ 30 years were analyzed, the results were similar to those for the whole study population (subject distribution = 20.7, 7.1, and 72.2% for Groups I, II, and III, respectively). Moreover, no difference in clinical characteristics was noted among the groups except in the prevalence of a history of aspirin intolerance (p < 0.01). Only three (9.7%) of 31 patients in Group I had a positive history of aspirin intolerance.
Baseline FEV1 did not differ significantly among the groups (mean values for Groups I-III: 84.5, 86.5, and 87.3%, respectively). However, the baseline FEV1 / forced vital capacity (FVC) ratios were significantly lower in Groups I (79.3 ± 1.5%) and II (78.3 ± 1.9%) than in Group III (82.7 ± 0.8%; both p < 0.05). The log methacholine-PC20 was significantly lower and the methacholine BRindex was significantly higher in Group I (log PC20: -0.24 ± 0.10; BRindex: 1.46 ± 0.02) than in Groups II (0.12 ± 0.16, p < 0.05; 1.36 ± 0.03, p < 0.01) and III (0.23 ± 0.07, p < 0.001; 1.37 ± 0.01, p < 0.001), respectively (Fig. 1). However, neither methacholine measurement was significantly different between Groups II and III. The distribution of the severity of methacholine AHR differed significantly among the groups (Fig. 2). The proportion of subjects with severe methacholine AHR to total patients was significantly higher in Group I than in the others (11/41 [26.8%] vs. 21/166 [12.7%], p < 0.05). However, it was not significantly different between the subjects with and without AIA (13/55 [23.6%] vs. 18/141 [12.8%], p = 0.061). In addition, both the methacholine-PC20 and BRindex were significantly correlated with the aspirin BRindex (Fig. 3).
The prevalence of pulmonary or extra-pulmonary symptoms that occurred in response to the inhalation aspirin challenge in patients with a positive response to the inhalation challenge (Group I) was significantly higher than in the others (Table 2). In addition, inhaled-aspirin-induced symptoms occurred more frequently in Group II than in Group III. However, seven patients symptomatic for inhalation, as well as the whole study population in Group II, showed no significant differences in methacholine responsiveness from Group III. In Group I, the FEV1 / FVC ratios in the patients who were symptomatic following the inhalation aspirin challenge were significantly lower than in those who were not (77.4 ± 1.5 vs. 83.6 ± 3.5%, p < 0.01). However, among patients with a negative response to the inhalation challenge, the symptomatic subjects showed no significant difference in clinical characteristics from the others.
Four patients received an oral challenge despite having a positive response to the inhalation challenge due to the absence of associated symptoms. Only one of these subjects showed a positive response to the oral challenge, suggesting false-positive responses to the inhalation challenge in many patients. The methacholine-PC20 was 0.74 mg/mL in a patient showing a positive oral challenge result compared with 0.09 and 0.12 mg/mL in two patients with a negative result. The remaining patient who showed a positive result on inhalation challenge but a negative result on oral challenge had a methacholine-PC20 > 25 mg/mL; however, even though his FEV1 30 minutes after the inhalation of 360 mg/mL aspirin decreased by 24.7% from the post-saline value, the FEV1 at 10 minutes decreased by only 16.2%, which was below the cutoff value (20%) for a positive response.
A relationship between AHR and aspirin can be inferred based on the fact that many patients with AIA are oral steroid dependent [16,17] and thus have severe AHR. This is the first study to definitively demonstrate a significant relationship between methacholine AHR and airway responses to an inhalation aspirin challenge. In this study, the methacholine-PC20 was significantly lower, whereas the methacholine-BRindex was significantly higher, in subjects with a positive response to the inhalation aspirin challenge than in the other groups. Moreover, airway responsiveness to methacholine was significantly correlated with that to aspirin. Our results also support a role for severe AHR in AIA by showing that a higher proportion of subjects with a positive inhalation challenge result had severe AHR compared to the proportions in the other groups. In previous studies [9-14], a relationship between AHR and AIA was not seen, suggesting that the number of subjects in these studies may have been too small (≤ 25) for the results to reach statistical significance.
Considering the natural history of AIA [17], patients with full-blown AIA who respond to the inhalation challenge may have had relatively severe AHR, whereas those with AIA who respond only to oral challenge may have had less severe AHR, similar to that of non-AIA patients. The responsiveness to methacholine in those subjects with a positive inhalation challenge result (Group I) was significantly different from that in those who showed a positive response to the oral challenge only (Group II), and no difference was found between Groups II and III even though the FEV1 / FVC ratio was significantly lower in Group II than in Group III. Thus, the prevalence of severe AHR was significantly higher in the AIA group than in the other groups when the inhalation challenge alone was used for the diagnosis of AIA, but not when the oral challenge was included. Nizankowska et al. [7] suggested that most patients with a positive response to oral challenge alone were considered positive on the basis of extra-bronchial symptoms. However, only 35.7% of the subjects in Group II were considered to show a positive oral challenge result on the basis of extrabronchial symptoms in the present study. Taken together, these observations suggest that patients with AIA who responded to the oral challenge alone may have had less severe AHR.
Although a significant relationship between methacholine AHR and the airway response to an inhalation aspirin challenge was found in this study, additional studies are required to determine whether aspirin intolerance is the cause of methacholine AHR or whether methacholine AHR results in a positive response to an inhalation aspirin challenge. As irritable airways constrict easily in response to various inhaled stimuli, the inhalation rather than ingestion of aspirin may affect airway responsiveness to the challenge. Therefore, to confirm the relationship between airway responsiveness to methacholine and to aspirin, it is necessary to use an oral aspirin challenge, even though almost all patients with positive inhalation responses also respond positively to the oral challenge [5,7]. In addition, a longitudinal study in which methacholine AHR is altered with time may be necessary to determine the extent to which AHR non-specifically affects inhalation aspirin challenge results, despite the repeatability of the inhalation aspirin challenge reported by Phillips et al. [11]. However, such efforts may not yield meaningful results because the underlying aspirin intolerance determined by an oral challenge can disappear with improvements in methacholine AHR due to asthma therapy [25].
Irritable airways may respond to inhaled aspirin non-specifically in asthma patients without aspirin intolerance. Our data supports this hypothesis because we found that three of four patients who received an oral challenge, despite having a positive response to the inhalation challenge due to the absence of associated symptoms, showed a negative response to the oral challenge. Furthermore, two negative oral responders had severe AHR. In another negative oral responder, FEV1 decreased in only one of the three measurements after the inhalation of 360 mg/mL aspirin to fulfill the positive criteria in which the lowest value was used as the representative result [4]. Therefore, it is possible that false-positive responses to aspirin occurred when the inhalation challenge was performed in patients with severe AHR who were asymptomatic to the inhalation challenge, or when the FEV1 decreased only transiently after the challenge. The high dose of inhaled aspirin used here does not pose a problem, as it is comparable to that reported by Phillips et al. [11], and recent guidelines [4] recommend as many as 43 breaths of 2 M (720 mg/mL) lysine-aspirin.
The rate of false-negative responses to the inhalation aspirin challenge was 25.5% in the present study. The maximum cumulative dose of aspirin (29.8 mg) used in this study may have resulted in false-negative results because it was only 16.4% of the 181.98 mg recommended by European guidelines [4] and used by Nizankowska et al. [7]. However, the sensitivity of the inhalation challenge reported by Nizankowska et al. [7] was only 77%, despite that strong extra-bronchial symptoms were added to a fall in FEV1 > 20% as indicating a positive response. Therefore, an additional oral challenge is desirable in cases in which the highest inhaled aspirin concentration fails to induce bronchospasms. The false-negative responders to the inhalation aspirin challenge complained of bronchial or extra-bronchial symptoms more frequently in this study. Thus, an oral challenge should be added for patients symptomatic to an inhalation challenge.
The AIA prevalence rate in the present study (28.1%) is consistent with that reported previously (14 to 29%) [1]. The factors mentioned above (i.e., underlying AHR, challenge method, positivity criteria, etc.) affect the prevalence of AIA. In addition, many patients with AIA are oral steroid dependent [16,17]; thus, an inhalation challenge cannot be performed due to airway instability [7]. Szczeklik et al. [17] reported that 15% of patients with AIA were unaware of their intolerance to aspirin and learned about it only after undergoing provocation testing. In the present study, 90.3% of the young adult male patients who required a medical certificate for exemption from obligatory military service had no previous history of aspirin intolerance. The most important reason for this discrepancy is that we examined aspirin intolerance routinely in these patients for the purpose of providing the necessary certificates.
In the present study, a considerable number of asthmatic subjects without a positive history of aspirin intolerance responded to the inhalation aspirin challenge. The airway response to aspirin was significantly related to methacholine AHR, and a false-positive response to the inhalation challenge would be expected in patients with severe AHR.
References
1. Jenkins C, Costello J, Hodge L. Systematic review of prevalence of aspirin induced asthma and its implications for clinical practice. BMJ 2004;328:434. PMID : 14976098.



2. Szczeklik A, Nizankowska-Mogilnicka E, Sanak M. In: Adkinson NF, Bochner BS, Busse WW, Holgate ST, Lemanske RF, Simons FE, eds. Hypersensitivity to aspirin and nonsteriodal anti-inflammatory drugs. Middleton's Allergy: Principle and Practice. 2009;7th ed. Philadelphia: Mosby-Elsevier Inc., 1227–1243.

3. Manning ME, Stevenson DD. Pseudoallergic drug reactions. Immunol Allergy Clin North Am 1991;11:659–678.

4. Nizankowska-Mogilnicka E, Bochenek G, Mastalerz L, et al. EAACI/GA2LEN guideline: aspirin provocation tests for diagnosis of aspirin hypersensitivity. Allergy 2007;62:1111–1118PMID : 17521312.


5. Dahlén B, Zetterström O. Comparison of bronchial and per oral provocation with aspirin in aspirin-sensitive asthmatics. Eur Respir J 1990;3:527–534PMID : 2376250.

6. Bianco S, Robuschi M, Petrigni G. Aspirin-induced tolerance in aspirin-induced asthma detected by a new challenge. Med Sci 1977;5:129–130.
7. Nizankowska E, Bestynska-Krypel A, Cmiel A, Szczeklik A. Oral and bronchial provocation tests with aspirin for diagnosis of aspirin-induced asthma. Eur Respir J 2000;15:863–869PMID : 10853850.


8. Melillo G, Bonini S, Cocco G, et al. EAACI provocation tests with allergens. Report prepared by the European Academy of Allergology and Clinical Immunology Subcommittee on provocation tests with allergens. Allergy 1997;52(35 Suppl):1–35PMID : 9224539.


9. Melillo G, Balzano G, Bianco S, et al. Report of the INTERASMA Working Group on Standardization of Inhalation Provocation Tests in Aspirin-induced Asthma. Oral and inhalation provocation tests for the diagnosis of aspirin-induced asthma. Allergy 2001;56:899–911PMID : 11551257.


10. Melillo G, Padovano A, Masi C, Melillo E, Cocco G. Aspirin-induced asthma and bronchial hyperresponsiveness. Allerg Immunol (Paris) 1991;23:423–427PMID : 1811646.

11. Phillips GD, Foord R, Holgate ST. Inhaled lysine-aspirin as a bronchoprovocation procedure in aspirin-sensitive asthma: its repeatability, absence of a late-phase reaction, and the role of histamine. J Allergy Clin Immunol 1989;84:232–241PMID : 2503553.


12. Croce M, Costa Manso E, Croce J, Gato JJ, Vargas F. Bronchial challenge with aspirin lysine in the diagnosis of asthmatics with sensitization to aspirin and its inhibition by aerosolized furosemide. J Investig Allergol Clin Immunol 1992;2:196–204.

13. Melillo G, Padovano A, Cocco G, Masi C. Dosimeter inhalation test with lysine acetylsalicylate for the detection of aspirin-induced asthma. Ann Allergy 1993;71:61–65PMID : 8328716.

14. Dahlén B, Melillo G. Inhalation challenge in ASA-induced asthma. Respir Med 1998;92:378–384PMID : 9692093.


15. Palikhe NS, Kim SH, Park HS. What do we know about the genetics of aspirin intolerance? J Clin Pharm Ther 2008;33:465–472PMID : 18834360.


16. Szczeklik A, Stevenson DD. Aspirin-induced asthma: advances in pathogenesis and management. J Allergy Clin Immunol 1999;104:5–13PMID : 10400832.


17. Szczeklik A, Nizankowska E, Duplaga M. Natural history of aspirin-induced asthma AIANE Investigators European Network on Aspirin-Induced Asthma. Eur Respir J 2000;16:432–436PMID : 11028656.


18. Weller PF. In: Adkinson NF, Bochner BS, Busse WW, Holgate ST, Lemanske RF, Simons FE, eds. Eosinophilia and eosinophil-related disorders. Middleton's Allergy: Principle and Practice. 2009;7th ed. Philadelphia: Mosby-Elsevier Inc., 859–877.

19. Sterk PJ, Fabbri LM, Quanjer PH, et al. Airway responsiveness. Standardized challenge testing with pharmacological, physical and sensitizing stimuli in adults Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal Official Statement of the European Respiratory Society. Eur Respir J 1993;6(16 Suppl):53–83PMID : 8425595.

20. Crapo RO, Casaburi R, Coates AL, et al. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med 2000;161:309–329PMID : 10619836.


21. Morris AH, Kanner RE, Crapo RO, Gardner RM. Clinical pulmonary function testing: a manual of uniform laboratory procedures. 1984;2nd ed. Salt Lake City: Intermountain Thoracic Society.
22. Woolcock AJ, Jenkins CR. Assessment of bronchial responsiveness as a guide to prognosis and therapy in asthma. Med Clin North Am 1990;74:753–765PMID : 2186241.


23. Burrows B, Sears MR, Flannery EM, Herbison GP, Holdaway MD. Relationships of bronchial responsiveness assessed by methacholine to serum IgE, lung function, symptoms, and diagnoses in 11-year-old New Zealand children. J Allergy Clin Immunol 1992;90(3 Pt 1):376–385PMID : 1527320.


24. Stevenson DD, Simon RA, Zuraw BL. In: Adkinson NF, Yunginger JW, Busse WW, Bochner BS, Holgate ST, Simons Fe, eds. Sensitivity to aspirin and nonsteriodal antiinflammatory drugs. Middleton's Allergy: Principle and Practice. 2003;6th ed. Philadelphia: Mosby, 1695–1710.
Figure 1
The methacholine-PC20 (A) and BRindex (B) for patients with asthma classified according to their responses to the inhalation and oral aspirin challenges. PC20, the provocative concentration of methacholine that resulted in a 20% decrease in the forced expiratory volume in one second (FEV1); BRindex, the bronchial reactivity index calculated using the equation: log10 (10 + maximal % fall in FEV1 / log10 [dose in mg/dL of methacholine required to produce the response]).
aPositive response to inhalation aspirin challenge.
bPositive response to oral aspirin challenge.
cNegative response to both inhalation and oral aspirin challenges.
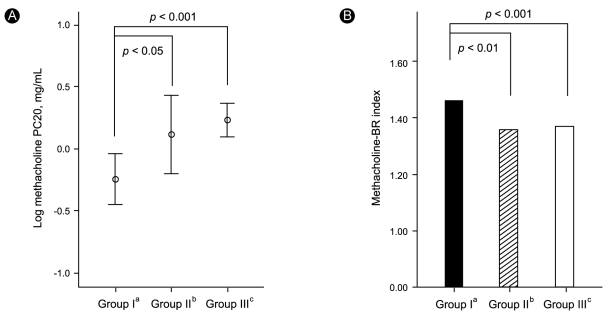
Figure 2
Distribution of the severity of methacholine airway hyperresponsiveness (AHR) in patients with asthma, classified according to their responses to the inhalation and oral aspirin challenges. The values of the provocative concentration of methacholine that resulted in a 20% decrease in the forced expiratory volume in one second (PC20) were divided as follows: severe, PC20 < 0.2; moderate, PC20 = 0.2 to 2.0; mild, PC20 = 2.0 to 16; and normal, PC20 > 16 mg/mL.
aPositive response to inhalation aspirin challenge.
bPositive response to oral aspirin challenge.
cNegative response to both inhalation and oral aspirin challenges.
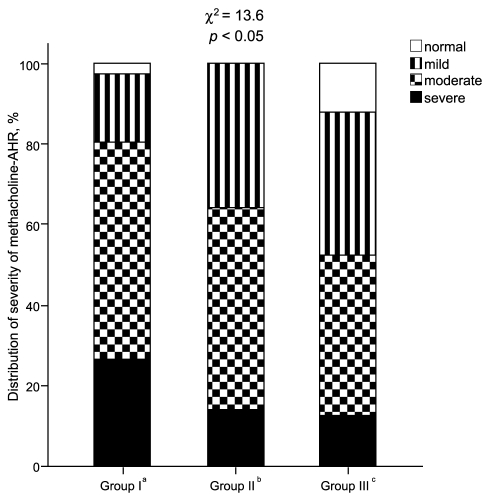
Figure 3
Relationship of airway responsiveness between the aspirin and methacholine bronchial reactivity index (BRindex), calculated using the equation: log10 (10 + maximal % fall in FEV1 / log10 [dose in mg/dL of stimulus required to produce the response]).

Table 1
Clinical characteristics of the asthmatic subjects classified according to their responses to the inhalation and oral aspirin challenges
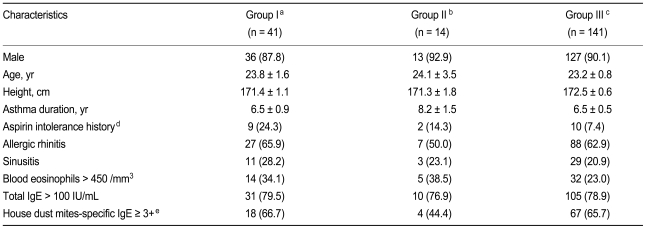
Values are presented as number (%) or mean ± SD.
An oral aspirin challenge was performed in cases of a negative response to the inhalation aspirin challenge.
aPositive response to inhalation aspirin challenge.
bPositive response to oral aspirin challenge.
cNegative response to both inhalation and oral aspirin challenges.
dp < 0.05 by χ2 analysis.
eThe levels of specific IgE for Dermatophagoides farinae and/or D. pteronyssinus were measured using the UniCAP®100ε system.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



