 |
 |
| Korean J Intern Med > Volume 25(4); 2010 > Article |
|
Abstract
Background/Aims
Clevudine, a pyrimidine nucleoside analogue, has potent antiviral effects in patients with chronic viral hepatitis B (CHB). We report the efficacy of initial treatment with clevudine in naïve patients with CHB living in Daejeon and Chungcheong Province, South Korea.
Methods
One hundred five adults with CHB were administered 30 mg of clevudine per day for an average of 51 weeks. We evaluated viral markers and liver biochemistry retrospectively every 3 months.
Results
Alanine aminotransferase (ALT), aspartate aminotransferase (AST), and hepatitis B virus (HBV) DNA before the treatment were 184 ± 188 IU/L, 150 ± 138 IU/L, and 7.1 ± 1.2 log copies/mL, respectively. Undetectable rates (< 60 IU/mL) of DNA were 36.2%, 68.9%, 83.6%, 76.2%, and 75.8% at 12, 24, 36, 48, and 60 weeks, respectively. Seroconversion rates were 9.1%, 13.6%, 24.6%, 26.5%, and 26.1% and ALT normalization rates were 64.5%, 78.1%, 87.9%, 90.0% at 12, 24, 36, and 48 weeks, respectively. Six patients (5.7%) had a viral breakthrough.
Hepatitis B virus (HBV) infection, which leads to chronic hepatitis and cirrhosis, may be responsible for life-threatening liver disease in some individuals, with 350 million carriers worldwide [1]. Substantial advances in oral nucleoside/nucleotide analogs have been made for treating chronic hepatitis B (CHB) in past decades. Nucleoside or nucleotide analogues competitively inhibit replication of HBV DNA through DNA polymerase. Lamivudine (LAM) is a first-generation oral nucleoside analogue for treating CHB. Previous studies in naïve patients found a cumulative LAM resistance incidence of 70 to 80% after 4 to 5 years of therapy [2,3]. Entecavir shows very low resistance and a potent antiviral effect in naïve patients with CHB. Although there is no evidence of carcinogenesis in human studies, this drug has produced some tumors such as adenoma of the lung, neurogenic tumors, and hepatocellular carcinoma in mice [4]. Clevudine is a nucleoside analogue of the unnatural L-configuration, which has potent antiviral activity against HBV [1,5]. Studies about the efficacy of initial treatment with clevudine in naïve chronic hepatitis B patients have been reported [1,6-11] and indicate that clevudine is very useful for treating CHB in the short term. However, these studies had short-term follow-ups of 12 or 24 weeks [1,6-11]. More long-term follow-up period studies are needed for evaluating the efficacy of clevudine treatment in patients with CHB. We prescribed clevudine to 105 naïve patients with CHB living in Daejeon and Chungcheong Province in South Korea for an average of 51 weeks.
This was a retrospective study and was conducted at six medical centers in Daejeon and Chungcheong Province, South Korea. Eligible patients were diagnosed with CHB with or without hepatitis B e antigen (HBeAg), HBV DNA levels above 1.0 × 105 copies/mL, > 2.0 × 104 IU/mL or > 0.4 pg/mL, and alanine aminotransferase (ALT) levels > 80 IU/L. All of the patients were prescribed clevudine for more than 6 months. The exclusion criteria were malignancy, alcoholism (> 20 g/day), severe fatty liver on ultrasonography, and a medication history of nucleoside or nucleotide analogues or interferon. The serum HBV DNA level was measured using the DNA PCR hybridization method (Roche, Indianapolis, IN, USA). The lower limit of HBV DNA detection was 300 copies/mL. Viral response was defined as < 300 copies/mL of HBV DNA. We defined viral breakthrough as a ten times rebound of HBV DNA copies compared with previous copies during treatment. Enrolled patients were prescribed 30 mg clevudine daily. We checked liver biochemistry, HBeAg/Ab, and HBV DNA every 12 weeks.
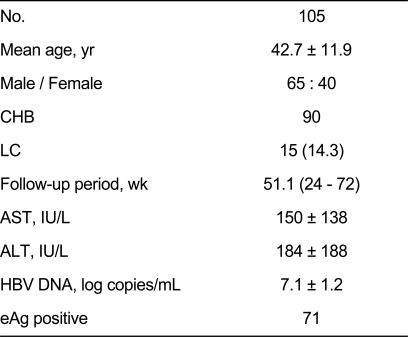
A total of 105 patients including 15 (14.3%) patients with liver cirrhosis were enrolled at six medical centers from February 2007 to August 2008. Seventy-one patients were HBeAg positive, and 34 patients were negative. The mean age was 42.7 years, and the mean follow-up period was 51.1 weeks. Median ALT at baseline was 184 IU/L, and median baseline HBV DNA was 7.1 log10 copies/mL (Table 1).
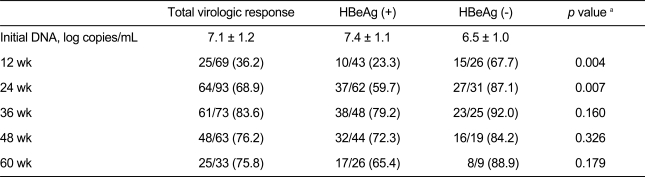
Viral response rates in all patients were 36.2%, 68.9%, 83.6%, 76.2%, and 75.8% at 12, 24, 36, 48, and 60 weeks, respectively. Viral response rates in HBeAg-positive patients were 23.3%, 59.7%, 79.2%, 72.3%, and 65.4%, and those in HBeAg-negative patients were 67.7%, 87.1%, 92.0%, 84.2%, and 88.9% at the corresponding weeks. The rates in HBeAg-positive patients were significantly lower at 12 and 24 weeks compared with those in HBeAg-negative patients. No significant difference was observed after 24 wks (Table 2).
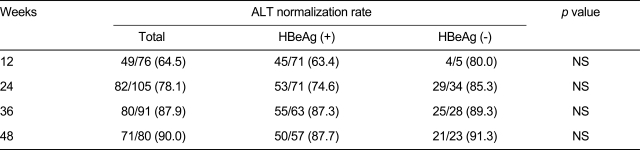
The serum ALT normalization rates in all patients were 78.1% and 90.0% at 24 and 48 weeks, respectively. The serum ALT normalization rates in HBeAg-positive versus HBeAg-negative patients were 63.4% vs. 80%, 74.6% vs. 85.3%, 87.3% vs. 89.3%, and 87.7% vs. 91.3% at 12, 24, 36, and 48 weeks, respectively (Table 3).
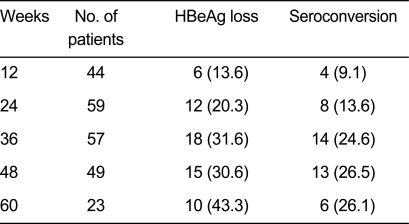
A total of 71e HBeAg-positive patients were enrolled. Among these patients, the HBeAg loss was 20.3%, 30.6%, and 43.3% at 24, 48, and 60 weeks, respectively, and the HBeAg seroconversion rates were 13.6%, 26.5%, and 26.1%, at 24, 48, and 60 weeks (Table 4).
The cumulative incidence rate of viral breakthrough in the HBeAg-positive versus HBeAg-negative patients was 1.4% vs. 0%, 3.3% vs. 3.4%, and 6.7% vs. 4.1% at 24, 36, and 48 weeks, respectively (Fig. 1). Six patients underwent treatment for viral breakthrough. Five cases were identified at 48 weeks, and one case at week 60. Five of these six patients underwent a tyrosine-methionine-aspartate-aspartate mutation (YMDD) examination, and four patients were positive (Table 5).
Clevudine is a nucleoside analog with an unnatural L-configuration. The mechanism by which clevudine exerts its antiviral effects is believed to be by the intracellular formation of the monophosphate, diphosphate, and triphosphate forms. Clevudine enters cells through both facilitated nucleoside transport and nonfacilitated passive diffusion and a substrate of three intracellular kinases is responsible for its phosphorylation [12].
When 21 patients received 30 mg clevudine for 24 weeks and were followed up for another 24 weeks off therapy, the clevudine treatment was well tolerated and resulted in more potent antiviral activity and a higher ALT normalization rate than a 12-week treatment, with durable efficacy at week 24 of therapy [1]. However, the experimental period in this study was less than 1 year. In another larger-scale study, 243 patients with HBeAg-positive CHB were randomized (3 : 1) to receive 30 mg clevudine once daily (n = 182) or placebo (n = 61) for 24 weeks. Patients were followed for a further 24 weeks off therapy. In that study, the 24-week clevudine therapy was well tolerated and showed a potent and sustained antiviral effect without evidence of viral resistance during the treatment period [9]. Here, we administered clevudine to 105 patients with CHB for about 1 year. As in other studies, clevudine showed powerful antiviral effects in this study [13]. With a limit of detection of < 300 copies/mL, the viral response rate was 76.2% (HBeAg-positive patients, 72.3%; HBeAg-negative patients, 84.2%) at 48 weeks. This result was remarkable enough to be compared with entecavir and tenofovir, which have negative seroconversion rates of 67% and 76%, respectively, after a 1-year treatment, but a direct comparison is difficult because of differences in the study methods and patient characteristics [9,10].
Viral response was highest at 36 weeks and subsequently decreased because some patients experienced viral breakthrough, and the HBV DNA PCR test at 48 or 60 weeks was not performed in some patients who had a good viral response.
The serum ALT normalization rate of all patients was 78.1% (HBeAg-positive patients, 74.6%; HBeAg-negative patients, 85.3%) and 90.0% (HBeAg-positive patients, 87.7%; HBeAg-negative patients, 91.3%) at 24 and 48 weeks, respectively. This result also shows similar efficacy to other antiviral agents. Approximately 6% of all patients with CHB showed viral breakthrough at 48 weeks. The viral breakthrough incidence rates were 2.9% and 7.0% for HBeAg-negative and -positive patients respectively; however, the difference was not statistically significant.
The cumulative viral breakthrough rate until week 48 was 6.3% (HBeAg-positive group, 7.0% [4/57]; HBeAg-negative group, 4.5% [1/22]) (Fig. 1).
Clevudine had powerful antiviral effects and had relatively lower viral breakthrough incidence rate compared with lamivudine (16-32% after 12 months); it is expected to replace lamivudine therapy [14]. When we performed the YMDD mutation test on five of six patients with viral breakthrough, the YMDD mutation was found in four patients. This suggested that clevudine cross-reacted with lamivudine. In particular, although our enrolled number of patients was insufficient to reach a definitive conclusion, clevudine appears to have been more effective for the HBeAg-negative group. Noticeably, although this study was not controlled, the HBeAg seroconversion rate at 48 weeks was 26.5% (13/49), which was relatively high compared with that for lamivudine (16-18%) [15]. A limitation of this study was that the examinations were not performed at regular intervals at all times, as it was a retrospective study and had a relatively short-term follow-up period of 1 year compared with other studies on medications that have been more fully researched. Furthermore, although no patient stopped clevudine because of myositis or others side effects, we did not measure serum creatine phosphokinase regularly or assess side effects. Despite these limitations, the results suggest that clevudine has the potential to be an effective therapeutic medication for patients with CHB; however, longer-term research will be needed for further evaluation.
References
1. Lee KS, Byun KS, Chung YH, et al. Clevudine therapy for 24 weeks further reduced serum hepatitis B virus DNA levels and increased ALT normalization rates without emergence of viral breakthrough than 12 weeks of clevudine therapy. Intervirology 2007;50:296–302PMID : 17622789.


2. Locarnini S. Molecular virology and the development of resistant mutants: implications for therapy. Semin Liver Dis 2005;25(Suppl 1):9–19PMID : 16103977.


3. Lok AS, Lai CL, Leung N, et al. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology 2003;125:1714–1722PMID : 14724824.


4. Matthews SJ. Entecavir for the treatment of chronic hepatitis B virus infection. Clin Ther 2006;28:184–203PMID : 16678641.


5. Koh KH, Kang CJ, Kim DH, et al. Development of clevudine resistance after switching from lamivudine in a patient with chronic hepatitis B. Korean J Gastroenterol 2008;52:325–328PMID : 19077481.

6. Liu SH, Grove KL, Cheng YC. Unique metabolism of a novel antiviral L-nucleoside analog, 2'-fluoro-5-methyl-beta-L-arabinofuranosyluracil: a substrate for both thymidine kinase and deoxycytidine kinase. Antimicrob Agents Chemother 1998;42:833–839PMID : 9559792.



7. Marcellin P, Mommeja-Marin H, Sacks SL, et al. A phase II dose-escalating trial of clevudine in patients with chronic hepatitis B. Hepatology 2004;40:140–148PMID : 15239097.


8. Lee HS, Chung YH, Lee K, et al. A 12-week clevudine therapy showed potent and durable antiviral activity in HBeAg-positive chronic hepatitis B. Hepatology 2006;43:982–988PMID : 16628625.


9. Yoo BC, Kim JH, Chung YH, et al. Twenty-four-week clevudine therapy showed potent and sustained antiviral activity in HBeAg-positive chronic hepatitis B. Hepatology 2007;45:1172–1178PMID : 17464992.


10. Yoo BC, Kim JH, Kim TH, et al. Clevudine is highly efficacious in hepatitis B e antigen-negative chronic hepatitis B with durable off-therapy viral suppression. Hepatology 2007;46:1041–1048PMID : 17647293.


11. Lim SG, Leung N, Hann HW, et al. Clinical trial: a phase II, randomized study evaluating the safety, pharmacokinetics and anti-viral activity of clevudine for 12 weeks in patients with chronic hepatitis B. Aliment Pharmacol Ther 2008;27:1282–1292PMID : 18363895.


12. Niu C, Murakami E, Furman PA. Clevudine is efficiently phosphorylated to the active triphosphate form in primary human hepatocytes. Antivir Ther 2008;13:263–269PMID : 18505177.



13. Kim MH, Kim KA, Lee JS, et al. Efficacy of 48-week clevudine therapy for chronic hepatitis B. Korean J Hepatol 2009;15:331–337PMID : 19783882.


Figure 1
Incidence rate of viral breakthrough according to treatment period. (A) Viral breakthrough rate of total patients. (B) Viral breakthrough rate of HBeAg (+) and HBeAg (-) patients.
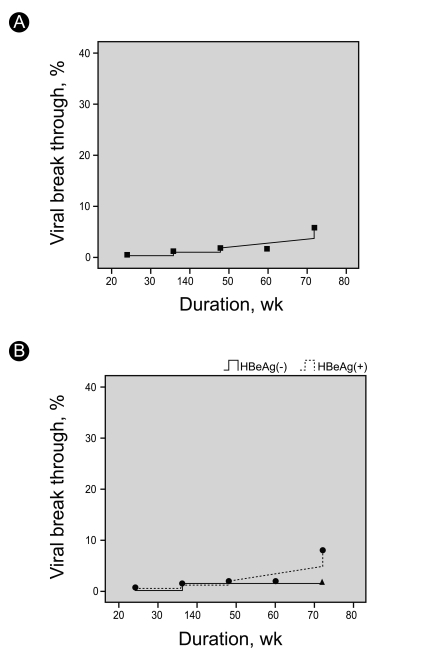
Table 2
Viral response rate of hepatitis B virus DNA and a comparison of HBeAg-positive and HBeAg-negative patients




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



