 |
 |
| Korean J Intern Med > Volume 26(1); 2011 > Article |
|
Abstract
The nuclear hormone receptor PPARγ is activated by several agonists, including members of the thiazolidinedione group of insulin sensitizers. Pleiotropic beneficial effects of these agonists, independent of their blood glucose-lowering effects, have recently been demonstrated in the vasculature. PPARγ agonists have been shown to lower blood pressure in animals and humans, perhaps by suppressing the renin-angiotensin (Ang)-aldosterone system (RAAS), including the inhibition of Ang II type 1 receptor expression, Ang-II-mediated signaling pathways, and Ang-II-induced adrenal aldosterone synthesis/secretion. PPARγ agonists also inhibit the progression of atherosclerosis in animals and humans, possibly through a pathway involving the suppression of RAAS and the thromboxane A2 system, as well as the protection of endothelial function. Moreover, PPARγ-agonist-mediated renal protection, especially the reduction of albuminuria, has been observed in diabetic nephropathy, including animal models of the disease, and in non-diabetic renal dysfunction. The renal protective activities may reflect, at least in part, the ability of PPARγ agonists to lower blood pressure, protect endothelial function, and cause vasodilation of the glomerular efferent arterioles. Additionally, anti-neoplastic effects of PPARγ agonists have recently been described. Based on the multiple therapeutic actions of PPARγ agonists, they will no doubt lead to novel approaches in the treatment of lifestyle-related and other diseases.
Peroxisome proliferator-activated receptor (PPAR)γ is a nuclear hormone receptor that, with the retinoid X receptor (RXR), binds as a heterodimer to the PPAR response element (PPRE), a direct repeat of 'AGGTCA' gapped by a nucleotide. PPAR is trans-activated by several agonists, including 15-deoxy-Δ12,14-prostaglandin J2 (15dPGJ2) and thiazolidinediones (TZDs); the latter are widely used as insulin-sensitizers in the treatment of diabetes [1] (Fig. 1). Recently, pleiotropic effects of PPARγ agonists in the vasculature have been demonstrated. These effects are independent of blood glucose-lowering activity and include protection against the progression of hypertension, atherosclerosis, and renal dysfunction [2]. In this review, we discuss recent findings regarding the additional beneficial aspects of PPARγ agonists in the vasculature, including conclusions based on our own data.
The blood-pressure-lowering effect of TZDs was recently demonstrated in a clinical study [4] and in the PROactive (PROspective pioglitAzone Clinical Trial In macroVascular Events) study, in which 5,238 type 2 diabetic patients were enrolled. Among the results of that trial, treatment with the TZD pioglitazone was shown to significantly decrease (3 mmHg) systolic blood pressure [5]. Because the renin-angiotensin (Ang)-aldosterone system (RAAS) plays the most important role in the progression of hypertension, we examined the effects of several PPARγ agonists on Ang II type 1 receptor (AT1R) expression in vascular smooth muscle cells (VSMCs). Interestingly, 15dPGJ2, as well as TZDs (pioglitazone, troglitazone, rosiglitazone), dose-dependently decreased the expression of AT1R mRNA [6,7].
Transcriptional analysis using the rat AT1R gene promoter (-1969/+104) and AT1R mRNA stability analysis using actinomycin D together revealed that PPARγ agonists decrease AT1R expression at the transcriptional level. Mutation analysis of the promoter demonstrated that transcriptional suppression was mediated within the -58/-34 region (TGC AGA GCA GCG ACG CCC CCT AGG C) of the AT1R gene promoter, which contains a GC-box-related sequence (underlined), but lacks a PPRE [6] (Fig. 2). Instead, the transcription factor Sp1 was shown to bind to and trans-activate the promoter region [6]. Overexpression of PPARγ and Sp1, followed by transcriptional analysis, electrophoretic mobility shift assay, and glutathione S-transferase pull-down assay, revealed that agonist-activated PPARγ does not bind to the -58/-34 region, but rather to Sp1 via a protein-protein interaction [6]. Moreover, Sp1 binding to the region was inhibited by coincubation with PPARγ [6]. These results suggested that PPARγ-agonist-induced transcriptional suppression of the AT1R gene is mediated by the inhibition of Sp1 binding to the -58/-34 region through a protein-protein interaction between agonist-activated PPARγ and Sp1 (Fig. 2). Furthermore, transcriptional suppression was abrogated by the over-expression of co-activator CERB-binding protein (CBP) and PPARγ phosphorylation by mitogen-activated protein (MAP) kinase [8], most likely due to the functional modification of PPARγ (Fig. 2). PPARγ-agonist-mediated suppression of AT1R expression was also demonstrated in Ang-II-infused rats [9,10]. Moreover, PPARγ agonists have been shown to suppress Ang-II-induced phosphatidylinositol 3-kinase and MAP kinase [10] and to ameliorate Ang-II-mediated inflammatory responses by interfering with the Toll-like-receptor-4-dependent signaling pathway [11]. Additionally, using human adrenal H295R cells, we recently found an inhibitory effect of PPARγ agonists on Ang-II-induced aldosterone synthase expression and aldosterone secretion [12]. Thus, PPARγ agonists not only down-regulate AT1R expression but also inhibit Ang-II-mediated signaling pathways and adrenal aldosterone synthesis/secretion, which, together, may result in RAAS suppression (Fig. 3). The ability of PPARγ agonists to lower blood pressure has been reported in Ang-II-infused Sprague-Dawley rats [9,10], spontaneously hypertensive rats [13], deoxycorticosterone acetate-salt rats [14], and hypertensive double-transgenic mice expressing human renin and human angiotensinogen transgenes [15]. Conversely, transgenic mice expressing a dominant-negative PPARγ P465L mutation exhibited hypertension [16], consistent with the phenotype of patients with an equivalent PPARγ P467L mutation [17], without affecting RAAS components. Moreover, genetic manipulation of mice with varying PPARγ expression demonstrated that blood pressure was lowered by an increase in receptor expression and increased when levels of the receptor were reduced [18]. Taken together, these results suggest that the decrease in blood pressure mediated by PPARγ agonists occurs through several different mechanisms in addition to RAAS inhibition.
Thromboxane (TX) A2, which is generated from prostaglandin H2, stimulates the contraction and proliferation of VSMCs and may be involved in the progression of atherosclerosis. We thus examined the effect of PPARγ agonists on the expression of TX synthase (TXS) in macrophages [19] and the TX receptor (TXR) in VSMCs [7,20]. PPARγ agonist suppressed both TXS and TXR expression at the transcriptional level [7,19,20]. Detailed analysis revealed that agonist-activated PPARγ inhibited nuclear factor E2-related factor 2 (NRF2) binding to DNA of the TXS gene [19], and Sp1 binding to DNA of the TXR gene [20], in both cases via protein-protein interactions. Accordingly, PPARγ agonists may suppress the progression of atherosclerosis through inhibition of both the TX system, including the synthesis and action/signal-transduction function of TXA2 (Fig. 3), and RAAS.
Atherosclerosis is usually preceded by endothelial dysfunction, whereas PPARγ agonists have been reported to improve the function of these cells not only in streptozotocin-induced diabetic rats [21] and diabetic db/db mice [22], but also in type 2 diabetic patients [23] and non-diabetic patients with coronary artery disease [24]. Additionally, transgenic mice specifically expressing dominant-negative PPARγ in endothelium developed endothelial dysfunction in response to a high-fat diet [25]. PPARγ agonists have also been reported to reduce carotid intimal-medial thickness (CIMT) and in-stent restenosis after coronary intervention in diabetic and non-diabetic patients [26], neointima formation after balloon injury in rats [27], and instent restenosis in atherosclerotic rabbits [28]. Metaanalysis of controlled trials involving type 2 diabetic patients found a significant reduction in CIMT and pulse wave velocity by PPARγ agonists of the TZD group [29]. We examined the direct effect of PPARγ agonists on endothelial gene expression by performing DNA microarray analyses. In those experiments, confluent human umbilical vein endothelial cells (HUVEC) were treated for 24 hours with the TZD pioglitazone, at a concentration (100 nM) mimicking the serum concentration in patients after a single oral administration. RNA extracted from the cells was processed for DNA microarray analyses using Human Genome Oligo Set (Operon Biotechnologies Inc., Huntsville, AL, USA), allowing the analysis of approximately 35,000 genes. Representative regulated genes are shown in Table 1. Among the genes induced by pioglitazone were tissue inhibitor of metalloproteinases-3, prostacyclin receptor, kallikrein 6 and 11, prostaglandin E2 receptor (EP1 subtype), and microsomal glutathione Stransferase 3. Suppressed genes included matrix metalloproteinase-10 and plasminogen activator inhibitor-2 [30]. The protection of endothelial function by PPARγ agonists may thus proceed through the regulation of gene expression. Recently, PPARγ agonists were reported to stimulate endothelial nitric oxide (NO) production in HUVECs [31] and to increase the number and function of endothelial progenitor cells in patients with coronary artery disease [32]. Additionally, disruption of the endothelium-specific PPARγ in mice resulted in the reduction of vascular NO production without affecting endothelial NO synthase expression [33]. These observations, in addition to our DNA microarray findings, may also explain the antiatherogenic effects of PPARγ agonists.
To examine the intra-renal localization of PPARγ protein, we generated an isoform-specific anti-PPARγ antibody, which was then used in the immunohistochemical analysis of Sprague-Dawley rat kidneys [34,35]. PPARγ protein was observed to be widely expressed in the nuclei of mesangial and epithelial cells in the glomeruli, proximal and distal tubules, loop of Henle, and medullary collecting ducts [34]. Additionally, the protein was detected in the intima/media of the renal vasculature [34]. We previously reported the vasodilating effects of the TZD troglitazone on the glomerular efferent arterioles of microdissected rabbit kidneys [36]. As suggested by the immunohistochemical data, these vasodilating effects may be mediated by PPARγ expressed in the intra-renal arterioles. The expression of PPARγ protein was also induced in distal tubules and cortical collecting ducts following administration of the TZD rosiglitazone to Sprague-Dawley rats [35]. These findings are potentially relevant in terms of pathophysiology, because TZDs have been reported to expand body fluid volume by the PPARγ-mediated stimulation of renal salt absorption through epithelial Na+ channels [37].
Renal protective effects of PPARγ ligands on type 2 diabetic patients with nephropathy, especially with respect to a reduction in urinary albumin, have recently been reported [38]. A meta-analysis of 15 studies involving 2,860 diabetic patients demonstrated a significant decrease in urinary albumin excretion in response to TZD-type PPARγ agonists [39]. Additionally, similar effects were observed in animal experiments using various rodent models of type 2 diabetes [38]. The mechanisms by which PPARγ agonists reduce urinary albumin remain unclear. However, together with their vasodilating effect on glomerular efferent arterioles [36], a lowering of blood pressure and an improvement of endothelial dysfunction may be cumulatively involved. Additionally, a recent study described the renal protective effect of PPARγ agonists against non-diabetic renal disease [40], indicating their general usefulness in the treatment of chronic kidney disease. We have also demonstrated a renal protective effect of the TZD rosiglitazone against cyclosporine-induced renal injury in Sprague-Dawley rats [41]. Moreover, the renal protective effect of the TZD pioglitazone against aging-related renal injury has been reported [42].
More than a decade has passed since the pleiotropic effects of PPARγ agonists were first reported. However, novel effects of PPARγ agonists are still being described on almost a monthly basis. In addition to the effects of these agents discussed in this review, anti-cancer activities of PPARγ agonist were recently reported [43]. We have also reported inhibitory effects of TZD-type PPARγ agonists on cell growth and REG (regenerating gene) Iα expression in gastrointestinal cancer cell lines [44]. It thus seems likely that the usefulness and effectiveness of PPARγ agonists against lifestyle-related diseases will be increasingly appreciated. This, in turn, may lead to further approved clinical applications of PPARγ agonists in the treatment of hypertension, atherosclerosis, and renal dysfunction, in addition to diabetes.
Acknowledgements
This work was supported by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan, Grants-in-Aid from the Ministry of Health, Labor, and Welfare of Japan, and Research Grants from Smoking Research Foundation.
References
1. Kliewer SA, Xu HE, Lambert MH, Willson TM. Peroxisome proliferator-activated receptors: from genes to physiology. Recent Prog Horm Res 2001;56:239–263PMID : 11237216.


2. Marchesi C, Schiffrin EL. Peroxisome proliferator-activated receptors and the vascular system: beyond their metabolic effects. J Am Soc Hypertens 2008;2:227–238PMID : 20409905.


3. Sugawara A, Uruno A, Kudo M, Matsuda K, Yang CW, Ito S. Effects of PPARγ on hypertension, atherosclerosis, and chronic kidney disease. Endocr J 2010;57:847–852PMID : 20890053.


4. Sarafidis PA, Lasaridis AN. Actions of peroxisome proliferator-activated receptors-gamma agonists explaining a possible blood pressure-lowering effect. Am J Hypertens 2006;19:646–653PMID : 16733240.


5. Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomized controlled trial. Lancet 2005;366:1279–1289PMID : 16214598.


6. Sugawara A, Takeuchi K, Uruno A, et al. Transcriptional suppression of type 1 angiotensin II receptor gene expression by peroxisome proliferator-activated receptor-gamma in vascular smooth muscle cells. Endocrinology 2001;142:3125–3134PMID : 11416035.


7. Sugawara A, Takeuchi K, Uruno A, et al. Differential effects among thiazolidinediones on the transcription of thromboxane receptor and angiotensin II type 1 receptor genes. Hypertens Res 2001;24:229–233PMID : 11409645.


8. Sugawara A, Takeuchi K, Uruno A, Kudo M, Sato K, Ito S. Effects of mitogen-activated protein kinase pathway and co-activator CREP-binding protein on peroxisome proliferator-activated receptor-gamma-mediated transcription suppression of angiotensin II type 1 receptor gene. Hypertens Res 2003;26:623–628PMID : 14567501.


9. Diep QN, El Mabrouk M, Cohn JS, et al. Structure, endothelial function, cell growth, and inflammation in blood vessels of angiotensin II-infused rats: role of peroxisome proliferator-activated receptor-gamma. Circulation 2002;105:2296–2302PMID : 12010913.


10. Benkirane K, Viel EC, Amiri F, Schiffrin EL. Peroxisome proliferator-activated receptor gamma regulates angiotensin II-stimulated phosphatidylinositol 3-kinase and mitogen-activated protein kinase in blood vessels in vivo. Hypertension 2006;47:102–108PMID : 16344371.


11. Ji Y, Liu J, Wang Z, Liu N, Gou W. PPARgamma agonist, rosiglitazone, regulates angiotensin II-induced vascular inflammation through the TLR4-dependent signaling pathway. Lab Invest 2009;89:887–902PMID : 19451898.


12. Uruno A, Matsuda K, Noguchi N, et al. Peroxisome proliferator-activated receptor-{gamma} suppresses CYP11B2 expression and aldosterone production. J Mol Endocrinol 2011;46:37–49PMID : 21106862.


13. Wu L, Wang R, De Champlain J, Wilson TW. Beneficial and deleterious effects of rosiglitazone on hypertension development in spontaneously hypertensive rats. Am J Hypertens 2004;17:749–756PMID : 15363815.


14. Iglarz M, Touyz RM, Amiri F, Lavoie MF, Diep QN, Schiffrin EL. Effect of peroxisome proliferator-activated receptor-alpha and -gamma activators on vascular remodeling in endothelin-dependent hypertension. Arterioscler Thromb Vasc Biol 2003;23:45–51PMID : 12524223.


15. Ryan MJ, Didion SP, Mathur S, Faraci FM, Sigmund CD. PPAR(gamma) agonist rosiglitazone improves vascular function and lowers blood pressure in hypertensive transgenic mice. Hypertension 2004;43:661–666PMID : 14744930.


16. Tsai YS, Kim HJ, Takahashi N, et al. Hypertension and abnormal fat distribution but not insulin resistance in mice with P465L PPARgamma. J Clin Invest 2004;114:240–249PMID : 15254591.



17. Barroso I, Gurnell M, Crowley VE, et al. Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature 1999;402:880–883PMID : 10622252.


18. Tsai YS, Xu L, Smithies O, Maeda N. Genetic variations in peroxisome proliferator-activated receptor gamma expression affect blood pressure. Proc Natl Acad Sci U S A 2009;106:19084–19089PMID : 19884495.



19. Ikeda Y, Sugawara A, Taniyama Y, et al. Suppression of rat thromboxane synthase gene transcription by peroxisome proliferator-activated receptor gamma in macrophages via an interaction with NRF2. J Biol Chem 2000;275:33142–33150PMID : 10930400.


20. Sugawara A, Uruno A, Kudo M, et al. Transcription suppression of thromboxane receptor gene by peroxisome proliferator-activated receptor-gamma via an interaction with Sp1 in vascular smooth muscle cells. J Biol Chem 2002;277:9676–9683PMID : 11777901.


21. Majithiya JB, Paramar AN, Balaraman R. Pioglitazone, a PPARgamma agonist, restores endothelial function in aorta of streptozotocin-induced diabetic rats. Cardiovasc Res 2005;66:150–161PMID : 15769458.


22. Miike T, Kunishiro K, Kanda M, Azukizawa S, Kurahashi K, Shirahase H. Impairment of endothelium-dependent ACh-induced relaxation in aorta of diabetic db/db mice: possible dysfunction of receptor and/or receptor-G protein coupling. Naunyn Schmiedebergs Arch Pharmacol 2008;377:401–410PMID : 18228001.


23. Martens FM, Visseren FL, de Koning EJ, Rabelink TJ. Short-term pioglitazone treatment improves vascular function irrespective of metabolic changes in patients with type 2 diabetes. J Cardiovasc Pharmacol 2005;46:773–778PMID : 16306801.


24. Staniloae C, Mandadi V, Kurian D, et al. Pioglitazone improves endothelial function in non-diabetic patients with coronary artery disease. Cardiology 2007;108:164–169PMID : 17077630.


25. Beyer AM, de Lange WJ, Halabi CM, et al. Endothelium-specific interference with peroxisome proliferator activated receptor gamma causes cerebral vascular dysfunction in response to a high-fat diet. Circ Res 2008;103:654–661PMID : 18676352.



26. Marx N, Walcher D. Vascular effects of PPARgamma activators: from bench to bedside. Prog Lipid Res 2007;46:283–296PMID : 17637478.


27. Lim S, Jin CJ, Kim M, et al. PPARgamma gene transfer sustains apoptosis, inhibits vascular smooth muscle cell proliferation, and reduces neointima formation after balloon injury in rats. Arterioscler Thromb Vasc Biol 2006;26:808–813PMID : 16424348.


28. Joner M, Farb A, Cheng Q, et al. Pioglitazone inhibits in-stent restenosis in atherosclerotic rabbits by targeting transforming growth factor-beta and MCP-1. Arterioscler Thromb Vasc Biol 2007;27:182–189PMID : 17068283.


29. Webb DR, Davies MJ, Gray LJ, et al. Searching for the right outcome? A systematic review and meta-analysis of controlled trials using carotid intima-media thickness or pulse wave velocity to infer antiatherogenic properties of thiazolidinediones. Diabetes Obes Metab 2010;12:124–132PMID : 19922476.


30. Kudo M, Sugawara A, Saito A, Uruno A, Ito S. Effects of pioglitazone, belaprost, and fluvastatin on gene expression in vascular endothelial cells revealed by DNA microarray analyses. J Hypertens 2006;24(Suppl 6):238.
31. Polikandriotis JA, Mazzella LJ, Rupnow HL, Hart CM. Peroxisome proliferator-activated receptor gamma ligands stimulate endothelial nitric oxide production through distinct peroxisome proliferator-activated receptor gamma-dependent mechanisms. Arterioscler Thromb Vasc Biol 2005;25:1810–1816PMID : 16020752.


32. Werner C, Kamani CH, Gensch C, Bohm M, Laufs U. The peroxisome proliferator-activated receptor-gamma agonist pioglitazone increases number and function of endothelial progenitor cells in patients with coronary artery disease and normal glucose tolerance. Diabetes 2007;56:2609–2615PMID : 17623816.


33. Kleinhenz JM, Kleinhenz DJ, You S, et al. Disruption of endothelial peroxisome proliferator-activated receptor-gamma reduces vascular nitric oxide production. Am J Physiol Heart Circ Physiol 2009;297:H1647–H1654PMID : 19666848.



34. Sato K, Sugawara A, Kudo M, Uruno A, Ito S, Takeuchi K. Expression of peroxisome proliferator-activated receptor isoform proteins in the rat kidney. Hypertens Res 2004;27:417–425PMID : 15253107.


35. Ahn KO, Lim SW, Yang HJ, et al. Induction of PPAR gamma mRNA and protein expression by rosiglitazone in chronic cyclosporine nephropathy in the rat. Yonsei Med J 2007;48:308–316PMID : 17461532.



36. Arima S, Kohagura K, Takeuchi K, et al. Biphasic vasodilator action of troglitazone on the renal microcirculation. J Am Soc Nephrol 2002;13:342–349PMID : 11805161.


37. Guan Y, Hao C, Cha DR, et al. Thiazolidinediones expand body fluid volume through PPARgamma stimulation of ENaC-mediated renal salt absorption. Nat Med 2005;11:861–866PMID : 16007095.


38. Yang J, Zhang D, Li J, Zhang X, Fan F, Guan Y. Role of PPARgamma in renoprotection in type 2 diabetes: molecular mechanisms and therapeutic potential. Clin Sci (Lond) 2009;116:17–26PMID : 19037881.


39. Sarafidis PA, Stafylas PC, Georgianos PI, Saratzis AN, Lasaridis AN. Effect of thiazolidinediones on albuminuria and proteinuria in diabetes: a meta-analysis. Am J Kidney Dis 2010;55:835–847PMID : 20110146.


40. Chung BH, Lim SW, Ahn KO, et al. Protective effect of peroxisome proliferator activated receptor gamma agonists on diabetic and non-diabetic renal diseases. Nephrology (Carlton) 2005;10(Suppl):S40–S43PMID : 16174287.


41. Chung BH, Li C, Sun BK, et al. Rosiglitazone protects against cyclosporine-induced pancreatic and renal injury in rats. Am J Transplant 2005;5:1856–1867PMID : 15996232.


42. Yang HC, Deleuze S, Zuo Y, Potthoff SA, Ma LJ, Fogo AB. The PPARgamma agonist pioglitazone ameliorates aging-related progressive renal injury. J Am Soc Nephrol 2009;20:2380–2388PMID : 19797472.



Figure 1
Schematic representation of PPARγ/RXR heterodimer binding to the PPRE on DNA. PPAR, peroxisome proliferator-activated receptor; RXR, retinoid X receptor; PPRE, PPAR response element. Modified figure from Sugawara et al. Endocr J 2010;57:847-852 with permission from The Japan Endocrine Society [3].
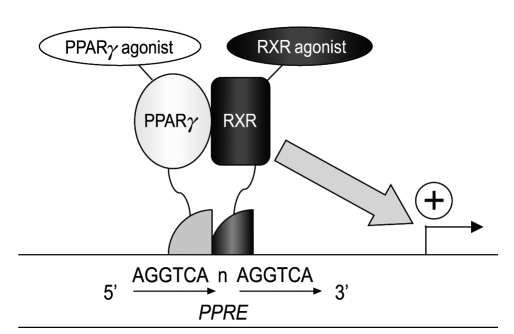
Figure 2
Possible mechanism of PPARγ-agonist-mediated transcriptional suppression of the AT1R gene promoter. Agonist-activated PPARγ may inhibit Sp1 binding to the GC-box-related sequence through a protein-protein interaction, which, in turn, would result in transcriptional suppression. The co-activator CBP and the phosphorylation of PPARγ by MAP kinase may together modulate PPARγ function. PPAR, peroxisome proliferator-activated receptor; AT1R, Ang II type 1 receptor; CBP, CERB-binding protein. Modified figure from Sugawara et al. Endocr J 2010;57:847-852 with permission from The Japan Endocrine Society [3].
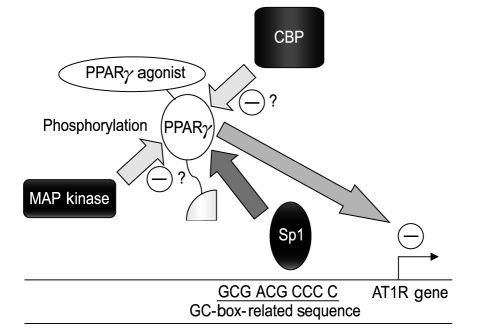
Figure 3
Possible effects of PPARγ agonists against atherosclerosis and hypertension through suppression of RAAS and the thromboxane system. PPAR, peroxisome proliferator-activated receptor; RAAS, renin-angiotensin (Ang)-aldosterone system; TX, thromboxane; TXS, TX synthase; TXR, TX receptor, VSMC: vascular smooth muscle cells. Modified figure from Sugawara et al. Endocr J 2010;57:847-852 with permission from The Japan Endocrine Society [3].
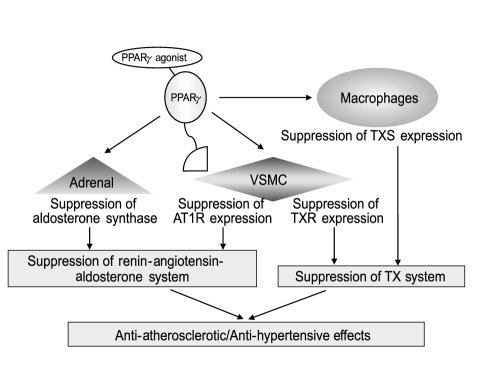
Table 1
Effects of PPARγ agonist pioglitazone on endothelial gene expression
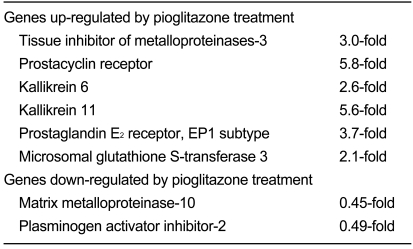
PPAR, peroxisome proliferator-activated receptor. Modified figure from Sugawara et al. Endocr J 2010;57:847-852 with permission from The Japan Endocrine Society [3].



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



