 |
 |
| Korean J Intern Med > Volume 26(3); 2011 > Article |
|
Abstract
Background/Aims
Methods
Results
Acknowledgments
References
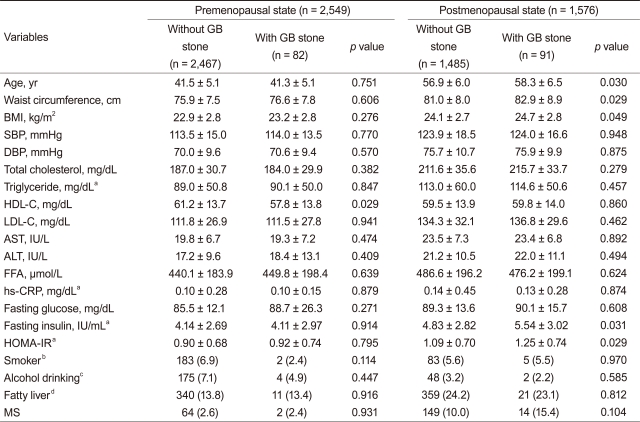
Table┬Ā1

Values are presented as mean ┬▒ SD or number (%).
GB, gallbladder; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL-C, high density lipoprotein-cholesterol; LDL-C, low density lipoprotein-cholesterol; AST, aspartate aminotransferase; ALT, alanine aminotransferase; FFA, free fatty acid; CRP, C-reactive protein; HOMA-IR, insulin resistance assessed by homeostasis model assessment; MS, metabolic syndrome.
aLog-transformed values were used for comparison.
bCurrent or past smoker.
c> 180 g/wk.
dAny degree of fatty infiltration on ultrasonography.
Table┬Ā2
Values are presented as mean ┬▒ SD or number (%).
GB, gallbladder; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL-C, high density lipoprotein-cholesterol; LDL-C, low density lipoprotein-cholesterol; AST, aspartate aminotransferase; ALT, alanine aminotransferase; FFA, free fatty acid; CRP, C-reactive protein; HOMA-IR, insulin resistance assessed by homeostasis model assessment; MS, metabolic syndrome.
aLog-transformed values were used for comparison.
bCurrent or past smoker.
c> 180 g/wk.
dAny degree of fatty infiltration on ultrasonography.
Table┬Ā3
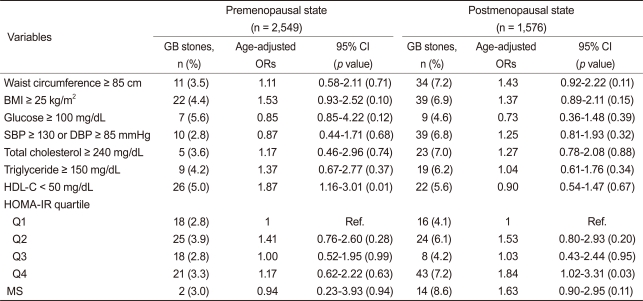
GB, gallbladder; CI, confidence interval; OR, odds ratio; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL-C, high density lipoprotein-cholesterol; HOMA-IR, insulin resistance assessed by homeostasis model assessment; Q, quartile; Ref., reference population group; MS, metabolic syndrome.
Table┬Ā4

OR, odds ratio; CI, confidence interval; BMI, body mass index; WC, waist circumference; HDL-C, high density lipoprotein-cholesterol; HOMA-IR, insulin resistance assessed by homeostasis model assessment.
aMultivariate models using the backward likelihood method were adjusted for age, smoking status, alcohol intake, body mass index, waist circumference, glucose, lipid profiles, blood pressure, and HOMA-IR as independent variables.
Table┬Ā5

GB, gallbladder; OR, odds ratio; CI, confidence interval; HOMA-IR, insulin resistance assessed by homeostasis model assessment; BMI, body mass index.
aMultivariate-adjusted ORs were adjusted for age, smoking status, alcohol intake, body mass index, waist circumference, glucose, lipid profiles, and blood pressure.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



