 |
 |
| Korean J Intern Med > Volume 27(1); 2012 > Article |
|
To the Editor,
Therapy-related acute myeloid leukemia (t-AML) typically occurs after exposure to alkylating agents, topoisomerase II inhibitors, other chemotherapeutics such as bimolane, or radiotherapy. Often, these secondary cancers have specific clinical and cytogenetic features [1]. The risk of t-AML development secondary to the use of antimetabolites alone is lower than that associated with the use of alkylating agents, although this risk remains higher than that in the general population. Although 5-fluorouracil (5-FU) is relatively frequently used in oncology, to our knowledge t-AML related to 5-FU has rarely been reported [2]. We present the case of a patient diagnosed with AML who previously received folinic acid (FA) and 5-FU as an adjuvant treatment for colon cancer following surgery.
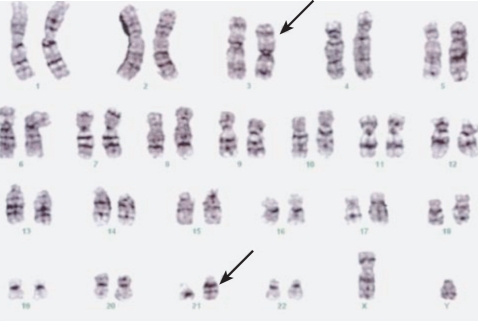
A 60-year-old man presented with general weakness and extreme fatigue. Forty months earlier, he had been diagnosed with stage IIB sigmoid colon cancer according to the American Joint Committee on Cancer (AJCC) staging system (T4N0M0). The mass was attached to the left diaphragm, and he received treatment with an extended left colectomy and subsequent radiation therapy (4,500 rad) of the left diaphragm in the 5 weeks following surgery. Following this, he received six cycles of adjuvant chemotherapy with FA and 5-FU (FA 20 mg/m2, 5-FU 425 mg/m2; D1-5 q4 weeks). Following this treatment, he remained disease free for 39 months while continuing on a daily regimen of doxifluridine (100 mg). On admission, the patient reported general weakness and dyspnea on exertion. He had no fever and no cervical, axillary, or inguinal lymphadenopathy. No hepatosplenomegaly or abnormal skin pigmentation was observed. Laboratory tests revealed a white cell count of 4.9 ├Ś 109/L, anemia (7.9 g/dL hemoglobin), and thrombocytopenia (platelet count 39 ├Ś 109/L). The differential showed 54% lymphocytes. Liver and kidney function tests were normal. The serum tumor marker, carcinoembryonic antigen (CEA), level was normal. No evidence of colon cancer recurrence was found by gastroscopy, colonoscopy, or whole-body positron emission tomography-computed tomography (PET-CT). The patient was referred to our department for the evaluation of pancytopenia. We performed a bone marrow biopsy and aspiration. The bone marrow examination showed many immature cells (20%) with a high nucleus/cytoplasm (N/C) ratio, sky-blue cytoplasm, and prominent nucleoli (Fig. 1). The myeloid series were normal in proportion but revealed a left shift. The erythroid series were normal in proportion, with increased monocytes. Cytogenetic analysis of the bone marrow disclosed 46,XY,t(3;21)(q26;q22) (Fig. 2). The patient was diagnosed with acute myelogenous leukemia. He was treated with induction chemotherapy consisting of idarubicin (12 mg/m2 D1-3) and ara-C (100 mg/m2 D1-7). He achieved complete remission following the induction chemotherapy. He continues to receive postremission chemotherapy with high-dose ara-C.
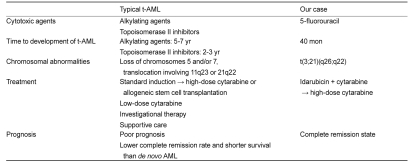
The term 'therapy-related leukemia' is descriptive and founded on a patient's history of exposure to cytotoxic agents. Though a causal relationship is suggested, the mechanism remains unproven. These neoplasms are thought to be the direct outcome of mutational events induced by cytotoxic therapy or via the selection of a myeloid clone with a mutator phenotype that has a remarkably elevated risk for a mutational event. Several distinct clinical and cytogenetic subtypes of t-AML are recognized; these are closely associated with the nature of the preceding treatment. The latency between primary diagnosis and therapy-related disease ranges from several months to a few years, depending in part on the cumulative dose or dose intensity of the precedent cytotoxic therapy, as well as on exposure to specific agents. Most patients have clonal chromosome abnormalities in their bone marrow cells at diagnosis [3]. Generally, 10-20% of all newly diagnosed cases of AML and myelodysplastic syndrome (MDS) are secondary to therapeutic regimens. A comparison of typical t-AML and our case is shown in Table 1. Clonal chromosomal abnormalities, often of a complex nature, are identified in most cases of classic therapy-related leukemia. Cytogenetic abnormalities, such as -5/del(5q), -7/del(7q), t(11q23), complex karyotypes, or +8 have been associated with t-AML. The most common single abnormality is monosomy 7(-7), followed in order of frequency by del(5q) and -5. Although these same abnormalities are observed in primary MDS and AML de novo, their frequency is clearly higher in therapy-related leukemia. Recent molecular examination has concentrated on identifying a presumptive leukemia suppressor gene in chromosome band 5q31, a critical region that is consistently deleted in leukemia cells with 5q abnormalities [4]. Secondary chromosomal changes, rather than primary abnormalities, leading to large-scale genomic imbalances are features of some forms of t-AML.
In our case, cy togenetic analysis showed t(3;21) (q26;q22). The t(3;21)(q26;q22) translocation is associated with myeloid leukemia and results in a chimeric oncoprotein containing AML1/RUNX1 alterably fused to EAP, MDS1, and/or EVL1. Activation of the AML1-/MDS1-/EVL1 fusion transcript through t(3;21) defines a very aggressive, therapy-related leukemic blast syndrome. Prior treatment with hydroxyurea or other antimetabolites is involved as a contributory cause. In a study performed by Yin et al. [5], the presence of t(3;21) heralded myeloid blast transformation in 14 patients with chronic myelogenous leukemia. Most patients had been treated with hydroxyurea in advance. Eight of 10 cases of t(3;21)-associated AML were secondary tumors following chemotherapy, including fludarabine and 5-FU in three patients each and etoposide in two patients.
Although the development of a second primary cancer cannot be ruled out, the presence of t(3:21)(q26:q22) and the prior use of 5-FU suggests t-AML due to 5-FU. Further study is needed to improve the therapeutic strategy for, and proper diagnosis of, t-AML.
References
1. Larson RA. Etiology and management of therapy-related myeloid leukemia. Hematology Am Soc Hematol Educ Program 2007;453ŌĆō459PMID : 18024664.


2. Turker A, Guler N. Therapy related acute myeloid leukemia after exposure to 5-fluorouracil: a case report. Hematol Cell Ther 1999;41:195ŌĆō196PMID : 10651118.


3. Smith SM, Le Beau MM, Huo D, et al. Clinical-cytogenetic associations in 306 patients with therapy-related myelodysplasia and myeloid leukemia: the University of Chicago series. Blood 2003;102:43ŌĆō52PMID : 12623843.


4. Le Beau MM, Albain KS, Larson RA, et al. Clinical and cytogenetic correlations in 63 patients with therapy-related myelodysplastic syndromes and acute nonlymphocytic leukemia: further evidence for characteristic abnormalities of chromosomes no. 5 and 7. J Clin Oncol 1986;4:325ŌĆō345PMID : 3950675.


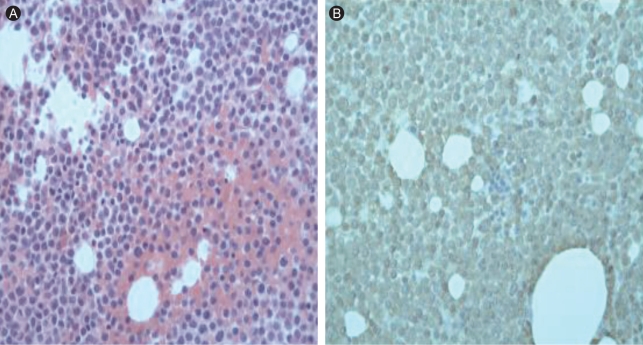
Figure┬Ā1
(A) Bone marrow biopsy revealed an increased proportion of immature myeloid cells (20%) with a high nucleus/cytoplasm ratio, basophilic cytoplasm, and conspicuous nucleoli. The myeloid series were normal in proportion but revealed a left shift. The erythroid series were normal in proportion, with increased monocytes (H&E, ├Ś 400). (B) Immature cells were diffusely positive for myeloperoxidase (MPO, ├Ś 400).



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



