 |
 |
| Korean J Intern Med > Volume 28(2); 2013 > Article |
|
Abstract
Background/Aims
Ozone is an environmentally reactive oxidant, and pycnogenol is a mixture of flavonoid compounds extracted from pine tree bark that have antioxidant activity. We investigated the effects of pycnogenol on reactive nitrogen species, antioxidant responses, and airway responsiveness in BALB/c mice exposed to ozone.
Methods
Antioxidant levels were determined using high performance liquid chromatography with electrochemical detection. Nitric oxide (NO) metabolites in bronchoalveolar lavage (BAL) fluid from BALB/c mice in filtered air and 2 ppm ozone with pycnogenol pretreatment before ozone exposure (n = 6) were quantified colorimetrically using the Griess reaction.
Results
Uric acid and ascorbic acid concentrations were significantly higher in BAL fluid following pretreatment with pycnogenol, whereas γ-tocopherol concentrations were higher in the ozone exposed group but were similar in the ozone and pycnogenol pretreatment groups. Retinol and γ-tocopherol concentrations tended to increase in the ozone exposure group but were similar in the ozone and pycnogenol pretreatment groups following ozone exposure. Malonylaldehyde concentrations increased in the ozone exposure group but were similar in the ozone and pycnogenol plus ozone groups. The nitrite and total NO metabolite concentrations in BAL fluid, which parallel the in vivo generation of NO in the airways, were significantly greater in the ozone exposed group than the group exposed to filtered air, but decreased with pycnogenol pretreatment.
The lung interfaces with the external environment and is frequently exposed to airborne pollutants such as ozone and particulates, and is prone to oxidant-mediated cellular damage [1-7]. The production of reactive nitrogen species (RNS) and reactive oxygen species (ROS) associated with oxidative stress are important factors in lung disease [2]. Ozone, a component of photochemical air pollution, is formed from volatile hydrocarbons, halogenated organics, and oxides of nitrogen in the presence of sunlight [2-7]. Ozone can react directly with unsaturated fatty acids and cell membranes to produce lipid ozonation products, which are small, diffusible, and relatively stable [8]. Ozone also leads to the oxidative modification of surfactant proteins, such as surfactant protein-A, which causes the lung to be more susceptible to lipid peroxidation and inflammation, and results in a reduction in phagocytosis [9]. Exposure of human airway epithelial cells to lipid ozonation products in vitro leads to the activation of eicosanoid metabolism, phospholipases A2, C, and D, and the induction of inflammatory mediators such as interleukin (IL)-6, IL-8, and prostaglandin E2 [10,11].
The dietary supplement pycnogenol is a water-soluble mixture of flavonoid compounds extracted from French maritime pine bark. It is utilized throughout the world as a phytochemical remedy for various diseases ranging from chronic inflammation to circulatory dysfunction. The flavonoids in pycnogenol have antioxidant properties [12,13] and may also act as modulators of metabolic enzymes [14-16] and other cellular functions [12,16,17]. Pycnogenol is a very potent antioxidant for scavenging ROS and RNS [16], has anti-inflammatory effects [18], may have efficient antioxidant activity [19-21], and shows some modulatory effects on the immune system [22].
Acute ozone exposure decreases pulmonary function, increases airway responsiveness, and induces airway inflammation [1-7,23-26]. There may be a common ozone adaptation mechanism that involves the regulation of ascorbic acid in the fluid that lines the lungs [27]. Antioxidant transport contributes to the maintenance of normal airway tone and reactivity under conditions of oxidative stress [28]. In the present study, we evaluated the effects of pycnogenol on ROS, RNS, and the antioxidant responses in BALB/c mice following acute ozone exposure.
Five- to 6-week-old female BALB/c mice, obtained from Daehan Laboratories (Daejeon, Korea), were maintained on ovalbumin-free diets. The mice were individually housed in rack-mounted stainless steel cages with free access to food and water. The mice housed in whole body exposure chambers were exposed to normal ozone concentrations of 0 (filtered room air) and 2 ppm for 3 hours (n = 6/group). Ozone was generated with Sander Model 50 ozonizers (Sander, Eltze, Germany). The concentration of ozone within the chambers was monitored throughout the exposure with ambient-air ozone motors (Model 49C, Thermo Environmental Instruments Inc., Franklin, MA, USA). Air sampling probes were placed in the breathing zone of the mice. The mean chamber ozone concentration (± SEM) during the 3-hour exposure period was 1.89 ± 0.06 ppm. The temperature and humidity were maintained at constant levels within the chamber. Pycnogenol was purchased from Horphag Research Ltd. (Guernsey, UK). The mice housed in whole body exposure chambers were treated with pycnogenol (100 mg/kg/day) orally for 5 days before ozone exposure. The study protocol was approved by the local research ethics committee of the Soonchunhyang University Bucheon Hospital research board.
An increase in the enhanced pause (Penh) was measured by barometric plethysmography using whole body plethysmography (Buxco, Troy, NY, USA) as an index of airway obstruction, immediately after ozone exposure while the animals were awake and breathing spontaneously [29]. Before taking readings, the box was calibrated by rapid injection of 150-µL air into the main chamber. The pressure differences between the main chamber of the whole body plethysmography-containing animal, and a reference chamber (box pressure signal) were measured. This box pressure signal is caused by changes in volume and resultant pressure in the main chamber during the respiratory cycle of the animal. A pneumotachograph with defined resistance in the wall of the main chamber acted as a low pass filter and allowed thermal compensation. The time constant of the box was determined to be approximately 0.02 seconds. Mice were placed in the main chamber, and baseline readings were taken over 3 minutes and averaged.
BAL was performed immediately after the last measurement of airway responsiveness. The mice were anesthetized intraperitoneally with 50 mg/kg pentobarbital sodium and were sacrificed by exsanguination from the abdominal aorta. The trachea was cannulated with a polyethylene tube through which the lungs were lavaged three times with 1-mL physiologic saline (4 mL total). The BAL fluid was filtered through a wet 4 × 4 gauze. Trypan blue exclusion for viability and total cell counts were performed. The BAL fluid was centrifuged at 150 ×g for 10 minutes. The pellet was immediately suspended in 4-mL physiological saline, and the total number of cells in the BAL fluid was counted in duplicate with a Neubauer improved hemocytometer. A 100-γL aliquot was centrifuged in a cytocentrifuge (Model 2 Cytospin, Shandon Scientific Co., Pittsburg, PA, USA). Differential cell counts were made from the centrifuged preparations stained with Diff-quick; at least ≥ 500 cells per animal were counted at a × 1,000 magnification using oil immersion.
BAL fluid was assayed for ascorbic acid, uric acid, retinol, γ-tocopherol, and γ-tocopherol. Malondialdehyde (MDA) was measured in lung tissue homogenates. Uric acid and ascorbic acid were determined simultaneously using reverse phase high performance liquid chromatography (HPLC) with electrochemical detection, based on the method of Mudway et al. [30]. HPLC determination of retinol, γ-tocopherol, and γ-tocopherol were based on the method of Bieri et al. [31] and described in detail for BAL fluid [32]. Lipid peroxidation, as a marker of oxidative damage, was determined based on the generation of thiobarbituric acid reactive substances in a 10-minute period and expressed as a concentration of MDA, based on the method of Ohkawa et al. [33]. Briefly, the reaction mixture containing 8% sodium dodecyl sulfate, 20% acetic acid (pH 4.0), and 0.8% thiobarbituric acid, was heated at 90℃ for 60 minutes. After cooling, an n-butanol and pyridine mixture (15:1, v/v) was added and centrifuged at 1,000 ×g for 10 minutes. The absorption of the supernatant was measured at 532 nm at room temperature using 1,1,3,3-tetramethoxypropane as an external standard.
Nitrite production was quantified colorimetrically after the Griess reaction as described by Greenberg et al. [34]. The BAL fluid supernatant, or a standard (100 µL), was combined with an equal volume of Griess reagent (1% sulfanilamide/0.1% naphthylethyllenedihydrochloride/2.5% phosphoric acid, Sigma Chemical Co., St. Louis, MO, USA) in duplicate in microtiter wells at room temperature. Chromophore absorbance at 540 nm was determined. The nitrite concentration was calculated using sodium nitrite (BDH Chemical Co., Poole, UK) as a standard. To assay nitrate, 200 µL of BAL fluid supernatant, or a standard containing 100-µL 200 mM ammonium formate (including 100 mM HEPES, Sigma Chemical Co.) was reduced to nitrite at 37℃ for 1 hour by adding 100 µL recombinant nitrate reductase (Escherichia coli [ATCC25922], American Type Collection, Rockville, MD, USA), followed by centrifugation to precipitate nonreacting E. coli for 5 minutes, after which the nitrite was quantified as described above.
All data were analyzed using SPSS version 14.0 for Windows (SPSS Inc., Chicago, IL, USA). Data are expressed as the means ± SEM. Intergroup comparisons were assessed using the nonparametric Mann-Whitney U test. A p value < 0.05 was considered to indicate statistical significance.
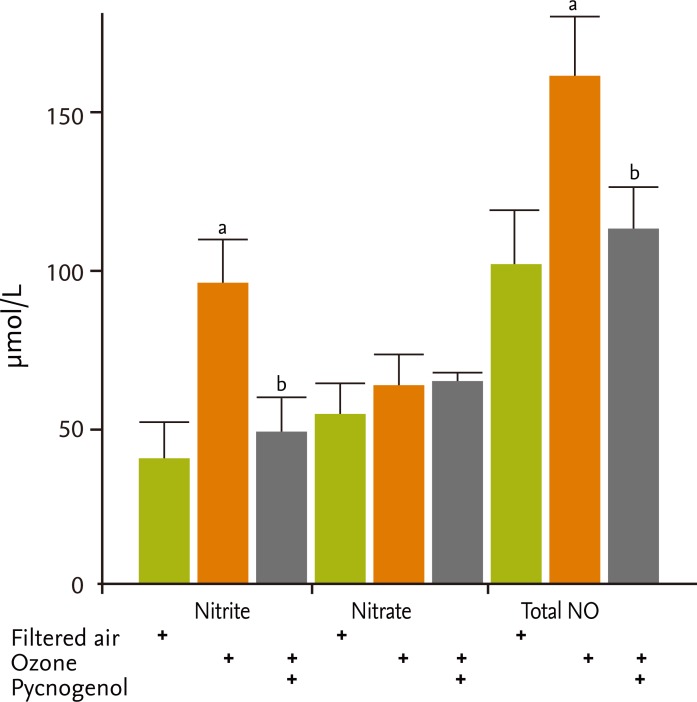
Concentrations of uric acid and ascorbic acid were significantly higher in BAL fluid following pretreatment with pycnogenol (uric acid, filtered air [4,831.9 ± 1,018.2 nmol/mg protein] vs. ozone [13,120.4 ± 2,798.7 nmol/mg protein] vs. pycnogenol plus ozone [21,139.2 ± 2,033.0 nmol/mg protein]; ascorbic acid [19,770.6 ± 4,551.2 nmol/mg protein] vs. ozone [25,058.3 ± 3,365.8 nmol/mg protein] vs. pycnogenol plus ozone [39,319.0 ± 5,010.0 nmol/mg protein], p < 0.05) (Fig. 1).
γ-tocopherol concentrations were higher in the ozone exposure group, but were similar in the ozone and pycnogenol pretreatment groups (filtered air [1,572.8 ±248.7 nmol/mg protein] vs. ozone [3,395.6 ± 248.7 nmol/mg protein] vs. pycnogenol plus ozone [3,097.9 ±273.0 nmol/mg protein]) (Fig. 1). Retinol and γ-tocopherol concentrations tended to increase in the ozone exposure group, but were similar in the ozone and pycnogenol pretreatment groups following ozone exposure. MDA concentrations were higher in the ozone exposure group but were similar in the ozone and pycnogerol plus ozone groups (filtered air [2.97 ± 0.08 nmol/mg protein] vs. ozone [3.29 ± 0.19 nmol/mg protein] vs. pycnogenol plus ozone [3.11 ± 0.05 nmol/mg protein]). The concentrations of nitrite and total nitric oxide (NO) metabolites in BAL fluid, which indicate the in vivo generation of NO in airways, were significantly greater in the ozone exposed group than in the group exposed to filtered air (nitrate metabolites in filtered air [40.8 ± 12.9 µmol/L] vs. ozone [98.0 ± 13.9 µmol/L], p < 0.05; total NO metabolites in filtered air [96.5 ± 20.3 µmol/L] vs. ozone [162.9 ± 16.4 µmol/L], p < 0.05), but decreased with pretreatment with pycnogenol (nitrate metabolites, 49.8 ± 11.1 µmol/L; NO metabolites, 116.0 ± 13.4 µmol/L; p < 0.05) (Fig. 2).
The recovery rates of BAL fluid were similar in all groups (2.65 ± 0.03 mL). Compared to that for filtered air, the proportion of neutrophils recovered in BAL fluid increased after exposure to 2 ppm ozone, and there were no differences between the ozone group and pycnogenol plus ozone group (filtered air [1.5% ± 0.21%] vs. ozone [12.10% ± 1.3%] vs. pycnogenol plus ozone [12.15% ± 1.65%], p < 0.01). The increases in Penh after ozone exposure were significantly reduced due to pretreatment with pycnogenol in mice that were exposed to 2 ppm ozone relative to the ozone exposure group (filtered air [0.75 ± 0.10] vs. ozone [1.81 ± 0.07] vs. pycnogenol plus ozone [1.44 ± 0.02]).
The results of this study indicate that pycnogenol functions as an antioxidant that reduces airway obstruction following ozone exposure and may modify RNS and antioxidants to minimize the effects of acute ozone exposure.
Oxidative stress is characterized by an imbalance between antioxidant defenses and damaging reactive species. The lungs have an extensive antioxidant network to protect against tissue damage by ROS and RNS [35-43]. ROS and RNS can regulate a diverse array of physiological processes, and deregulation of oxidant signaling may cause or accelerate a host of pathological conditions as it is an important regulator of physiological and pathophysiological outcomes [37]. The lungs have large epithelial surface areas that are exposed to inhaled airborne reactive pollutants and a multitude of airborne microorganisms, which makes them particularly susceptible to environmental oxidant-mediated injury. Metabolic reactions and environmental pollutants such as ozone, cigarette smoke, and particulate matter can produce an oxidizing lung environment, leading to endogenous and exogenous production of ROS [38]. Ozone imposes an oxidative burden on the lung directly as an oxidant during exposure, and indirectly by inducing inflammation. A single, acute exposure to ozone modifies the protective antioxidant defense network in the respiratory tract, with consumption of ascorbic acid and uric acid and reduced glutathione levels [26].
Protective compounds include small antioxidant molecules such as vitamin C, vitamin E, uric acid, the tripeptide glutathione, mucins, metal binding proteins such as transferrin, lactoferrin, and metallothioneins, and a variety of enzymes, such as superoxide dismutases, glutathione dependent enzymes, catalase, and various thiol containing proteins that play an important role in protection against ROS [35-43]. The balance between oxidative burden and the body's antioxidant potential in the pathogenesis of airway obstruction has been the focus of recent investigations. Antioxidants in fluids that coat the lung epithelium constitute an initial line of defense against inhaled environmental oxidants such as ozone, nitrogen oxides, and tobacco smoke. Ozone exposure is associated with adverse respiratory effects, in particular with reductions in lung function [44].
Pycnogenol is a mixture of compounds extracted from the bark of pine trees (Pinus maritima) whose chromatographic profile is composed of phenolic, procyanidin/proanthocyanidin, and flavonoid compounds existing as monomers, dimers, and oligomers of five to seven units [12]. The oligomers are composed mainly of catechin and epicatechin units linked together by four to eight or four to six bonds [12,45]. Other minor constituents of pycnogenol include phenolic acids, glucosides, and glucose esters [12,45]. Pycnogenol lacks toxicity (pharmacologic LD50, 3 g/kg) and is nonteratogenic and nonmutagenic [46]. Pycnogenol is an efficient scavenger of ROS. Indeed, HO· and the superoxide radical anion (O2·-) scavenging activity are maintained after treatment with ascorbate oxidase, indicating that ascorbate, which may be present in the mixture, is responsible for the antioxidant activity. On the other hand, ultrafiltration affects O2·- scavenging activity, suggesting a contribution to the antioxidant activity of high molecular weight compounds present in the mixture [47,48].
Because there are limited data on the effects of pycnogenol and its mechanisms of action, we investigated its effects on RNS, as well as its antioxidant responses and airway responsiveness, following ozone exposure. Our data suggest that pycnogenol has a heightened antioxidant response in mice following ozone exposure. In accordance with previous studies [24,25,44], we found that although the number of neutrophils in BAL did not decrease, increased airway responsiveness following ozone exposure was decreased following pycnogenol treatment after ozone exposure, suggesting that the effect of pycnogenol be not sufficient for inflammatory cells and it could be effective for airway hyperresponsiveness via antioxidant mechanisms.
Antioxidant vitamins are free radical scavengers that can protect against photo oxidant exposure. Vitamins C and E are powerful antioxidants found in the lung that protect against oxidative damage [49]. Although vitamin E is predominantly membrane bound, there is a close interaction between vitamins C and E [50], because vitamin C not only functions directly as an antioxidant but also recycles the antioxidant capacity of oxidized vitamin E [51]. Taking a daily supplement (75 mg vitamin E, 650 mg vitamin C, 15 mg β-carotene) may have a residual protective effect on the lung [52]. Indeed, diet affords protection against ozone induced oxidant toxicity. Protection is mediated partially by increases in ascorbic acid in the fluid bathing the lung surface, thereby providing an antioxidant sink that minimizes the ability of ozone to reach biological targets [53]. In the present study, although uric acid was increased in BAL fluid following ozone exposure and was increased further in BAL fluid following administration of pycnogenol, our data suggest that uric acid may be a protective antioxidant and a marker of the effectiveness of pycnogenol. MDA in BAL fluid is a marker of oxidative damage [32]. In the present study, MDA levels did not change following pycnogenol administration, indicating that MDA may be a less valuable marker of antioxidant effects following ozone exposure.
Few studies have investigated antioxidant levels in BAL fluid in relation to lung function [54,55]. Antioxidant vitamins may play a role in respiratory health; vitamin E and β-cryptoxanthin appear to be stronger correlates of lung function than other antioxidant vitamins. Considerable uncertainty about the association between BAL fluid antioxidants and lung function remains [55]. In the present study, airway responsiveness following pycnogenol treatment was decreased and the uric acid level was increased, suggesting that pycnogenol may increase the antioxidant response and decrease airway obstruction following oxidative ozone exposure. In conclusion, our data suggest that pretreatment with pycnogenol before ozone exposure may mitigate airway obstruction and the oxidizing effects of ozone.
1. The lungs have an extensive antioxidant network to protect against tissue damage from reactive oxygen and nitrogen species.
2. Ozone imposes an oxidative burden on the lung through direct oxidant exposure and by indirectly inducing inflammation.
3. Pycnogenol may mitigate airway obstruction and the oxidizing effects of ozone.
Acknowledgements
This research was supported by the Korea Ministry of Environment (2012001360001) Environmental Health Action Program.
References
1. Jang AS, Yeum CH, Son MH. Epidemiologic evidence of a relationship between airway hyperresponsiveness and exposure to polluted air. Allergy 2003;58:585–588PMID : 12823115.


2. Jang AS, Choi IS, Yang SY, et al. Antioxidant responsiveness in BALB/c mice exposed to ozone. Respiration 2005;72:79–84PMID : 15753639.


3. Jang AS, Choi IS, Lee JU, Park SW, Lee JH, Park CS. Changes in the expression of NO synthase isoforms after ozone: the effects of allergen exposure. Respir Res 2004;5:5. PMID : 15251042.



4. Jang AS, Choi IS, Kim SW, Song BC, Yeum CH, Jung JY. Airway obstruction after acute ozone exposure in BALB/c mice using barometric plethysmography. Korean J Intern Med 2003;18:1–5PMID : 12760261.



5. Jang AS, Choi IS, Lee JU. Neuronal nitric oxide synthase is associated with airway obstruction in BALB/c mice exposed to ozone. Respiration 2003;70:95–99PMID : 12584398.


6. Jang AS, Choi IS, Koh YI, Park CS, Lee JS. The relationship between alveolar epithelial proliferation and airway obstruction after ozone exposure. Allergy 2002;57:737–740PMID : 12121195.


7. Jang AS, Choi IS, Lee JH, Park CS. Prolonged ozone exposure in an allergic airway disease model: adaptation of airway responsiveness and airway remodeling. Respir Res 2006;7:24. PMID : 16472404.



8. Leikauf GD, Zhao Q, Zhou S, Santrock J. Ozonolysis products of membrane fatty acids activate eicosanoid metabolism in human airway epithelial cells. Am J Respir Cell Mol Biol 1993;9:594–602PMID : 8257591.


9. Mikerov AN, Umstead TM, Gan X, et al. Impact of ozone exposure on the phagocytic activity of human surfactant protein A (SP-A) and SP-A variants. Am J Physiol Lung Cell Mol Physiol 2008;294:L121–L130PMID : 17981957.



10. Kafoury RM, Pryor WA, Squadrito GL, Salgo MG, Zou X, Friedman M. Induction of inflammatory mediators in human airway epithelial cells by lipid ozonation products. Am J Respir Crit Care Med 1999;160:1934–1942PMID : 10588609.


11. Kafoury RM, Pryor WA, Squadrito GL, Salgo MG, Zou X, Friedman M. Lipid ozonation products activate phospholipases A2, C, and D. Toxicol Appl Pharmacol 1998;150:338–349PMID : 9653065.


12. Packer L, Rimbach G, Virgili F. Antioxidant activity and biologic properties of a procyanidin-rich extract from pine (Pinus maritima) bark, pycnogenol. Free Radic Biol Med 1999;27:704–724PMID : 10490291.


13. Rice-Evans CA, Miller NJ. Antioxidant activities of flavonoids as bioactive components of food. Biochem Soc Trans 1996;24:790–795PMID : 8878849.


14. Boege F, Straub T, Kehr A, et al. Selected novel flavones inhibit the DNA binding or the DNA religation step of eukaryotic topoisomerase I. J Biol Chem 1996;271:2262–2270PMID : 8567688.


15. Koley AP, Buters JT, Robinson RC, Markowitz A, Friedman FK. Differential mechanisms of cytochrome P450 inhibition and activation by alpha-naphthoflavone. J Biol Chem 1997;272:3149–3152PMID : 9013547.


16. De Azevedo WF Jr, Mueller-Dieckmann HJ, Schulze-Gahmen U, Worland PJ, Sausville E, Kim SH. Structural basis for specificity and potency of a flavonoid inhibitor of human CDK2, a cell cycle kinase. Proc Natl Acad Sci U S A 1996;93:2735–2740PMID : 8610110.



17. Moini H, Guo Q, Packer L. Enzyme inhibition and protein-binding action of the procyanidin-rich french maritime pine bark extract, pycnogenol: effect on xanthine oxidase. J Agric Food Chem 2000;48:5630–5639PMID : 11087530.


18. Saliou C, Rimbach G, Moini H, et al. Solar ultraviolet-induced erythema in human skin and nuclear factor-kappa-B-dependent gene expression in keratinocytes are modulated by a French maritime pine bark extract. Free Radic Biol Med 2001;30:154–160PMID : 11163532.


19. Ueda T, Armstrong D. Preventive effect of natural and synthetic antioxidants on lipid peroxidation in the mammalian eye. Ophthalmic Res 1996;28:184–192PMID : 8829176.


20. van Jaarsveld H, Kuyl JM, Schulenburg DH, Wiid NM. Effect of flavonoids on the outcome of myocardial mitochondrial ischemia/reperfusion injury. Res Commun Mol Pathol Pharmacol 1996;91:65–75PMID : 8824932.

21. Rong Y, Li L, Shah V, Lau BH. Pycnogenol protects vascular endothelial cells from t-butyl hydroperoxide induced oxidant injury. Biotechnol Ther 1994;5:117–126PMID : 8608322.

22. Cheshier JE, Ardestani-Kaboudanian S, Liang B, et al. Immunomodulation by pycnogenol in retrovirus-infected or ethanol-fed mice. Life Sci 1996;58:PL87–PL96.

23. Committee of the Environmental and Occupational Health Assembly of the American Thoracic Society. Health effects of outdoor air pollution. Am J Respir Crit Care Med 1996;153:3–50PMID : 8542133.


24. Holtzman MJ, Fabbri LM, O'Byrne PM, et al. Importance of airway inflammation for hyperresponsiveness induced by ozone. Am Rev Respir Dis 1983;127:686–690PMID : 6859651.

25. Gordon T, Taylor BF, Amdur MO. Ozone inhibition of tissue cholinesterase in guinea pigs. Arch Environ Health 1981;36:284–288PMID : 7316565.


26. Kelly FJ, Blomberg A, Frew A, Holgate ST, Sandstrom T. Antioxidant kinetics in lung lavage fluid following exposure of humans to nitrogen dioxide. Am J Respir Crit Care Med 1996;154(6 Pt 1):1700–1705PMID : 8970358.


27. Wiester MJ, Winsett DW, Richards JH, Jackson MC, Crissman KM, Costa DL. Ozone adaptation in mice and its association with ascorbic acid in the lung. Inhal Toxicol 2000;12:577–590PMID : 10880145.


28. Freed AN, Cueto R, Pryor WA. Antioxidant transport modulates peripheral airway reactivity and inflammation during ozone exposure. J Appl Physiol 1999;87:1595–1603PMID : 10562596.


29. Jacky JP. A plethysmograph for long-term measurements of ventilation in unrestrained animals. J Appl Physiol 1978;45:644–647PMID : 101497.


30. Mudway IS, Housley D, Eccles R, et al. Differential depletion of human respiratory tract antioxidants in response to ozone challenge. Free Radic Res 1996;25:499–513PMID : 8951423.


31. Bieri JG, Poukka RK, Prival EL. Determination of alpha-tocopherol in erythrocytes by gas-liquid chromatography. J Lipid Res 1970;11:118–123PMID : 5418474.


32. Blomberg A, Mudway IS, Nordenhall C, et al. Ozone-induced lung function decrements do not correlate with early airway inflammatory or antioxidant responses. Eur Respir J 1999;13:1418–1428PMID : 10445622.


33. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979;95:351–358PMID : 36810.


34. Greenberg SS, Xie J, Spitzer JJ, et al. Nitro containing L-arginine analogs interfere with assays for nitrate and nitrite. Life Sci 1995;57:1949–1961PMID : 7475944.


35. Bast A, Weseler AR, Haenen GR, den Hartog GJ. Oxidative stress and antioxidants in interstitial lung disease. Curr Opin Pulm Med 2010;16:516–520PMID : 20592594.


36. Finkel T. Signal transduction by reactive oxygen species. J Cell Biol 2011;194:7–15PMID : 21746850.



37. Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell 2005;120:483–495PMID : 15734681.


38. Comhair SA, Erzurum SC. Redox control of asthma: molecular mechanisms and therapeutic opportunities. Antioxid Redox Signal 2010;12:93–124PMID : 19634987.



39. Kliment CR, Oury TD. Oxidative stress, extracellular matrix targets, and idiopathic pulmonary fibrosis. Free Radic Biol Med 2010;49:707–717PMID : 20452419.


40. Wood LG, Wark PA, Garg ML. Antioxidant and anti-inflammatory effects of resveratrol in airway disease. Antioxid Redox Signal 2010;13:1535–1548PMID : 20214495.


41. Park HS, Kim SR, Lee YC. Impact of oxidative stress on lung diseases. Respirology 2009;14:27–38PMID : 19144046.


42. Cho YS, Moon HB. The role of oxidative stress in the pathogenesis of asthma. Allergy Asthma Immunol Res 2010;2:183–187PMID : 20592917.



43. Nadeem A, Masood A, Siddiqui N. Oxidant: antioxidant imbalance in asthma: scientific evidence, epidemiological data and possible therapeutic options. Ther Adv Respir Dis 2008;2:215–235PMID : 19124374.


44. Macnee W, Rahman I. Oxidants and antioxidants as therapeutic targets in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999;160(5 Pt 2):S58–S65PMID : 10556172.


45. Rohdewald P. In: Rice-Evans C, Packer L, eds. Pycnogenol. Flavonoids in Health and Disease. 1998;New York: Marcel Dekker, 405–419.
46. Masquelier J . Plant extract with a proanthocyanidins content as therapeutic agent having radical scavenging effect and use thereof. United States patent US 4,698,360. 1987. 10. 06.
47. Blazso G, Gabor M, Sibbel R, Rohewald P. Antiinflammatory and superoxide radical scavenging activities of procyanidins containing extract from the bark of Pinus pinaster Sol. and its fractions. Pharmaceut Pharmacol Lett 1994;3:217–220.
48. Virgili F, Kobuchi H, Packer L. Procyanidins extracted from Pinus maritima (Pycnogenol): scavengers of free radical species and modulators of nitrogen monoxide metabolism in activated murine RAW 264.7 macrophages. Free Radic Biol Med 1998;24:1120–1129PMID : 9626566.


49. Heffner JE, Repine JE. Pulmonary strategies of antioxidant defense. Am Rev Respir Dis 1989;140:531–554PMID : 2669581.


50. Burton GW, Joyce A, Ingold KU. First proof that vitamin E is major lipid-soluble, chain-breaking antioxidant in human blood plasma. Lancet 1982;2:327. PMID : 6124736.

51. McCay PB. Vitamin E: interactions with free radicals and ascorbate. Annu Rev Nutr 1985;5:323–340PMID : 2992548.


52. Romieu I, Meneses F, Ramirez M, et al. Antioxidant supplementation and respiratory functions among workers exposed to high levels of ozone. Am J Respir Crit Care Med 1998;158:226–232PMID : 9655734.


53. Kari F, Hatch G, Slade R, Crissman K, Simeonova PP, Luster M. Dietary restriction mitigates ozone-induced lung inflammation in rats: a role for endogenous antioxidants. Am J Respir Cell Mol Biol 1997;17:740–747PMID : 9409561.


-
METRICS

- Related articles
-
Analgesic effect of quetiapine in a mouse model of cancer-induced bone pain2017 November;32(6)




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


