See Article on Page 314-321
It has been accepted since the 1990s that palliative chemotherapy can significantly prolong the survival of patients with advanced gastric carcinoma, compared to supportive care alone [1,2]. However, there is controversy over the benefit of salvage therapy past second-line due to the lack of evidence. Recently, there has been renewed interest in salvage chemotherapy after first- and second-line treatments have failed because of the prolonged survival time and relatively low toxicity of agents.
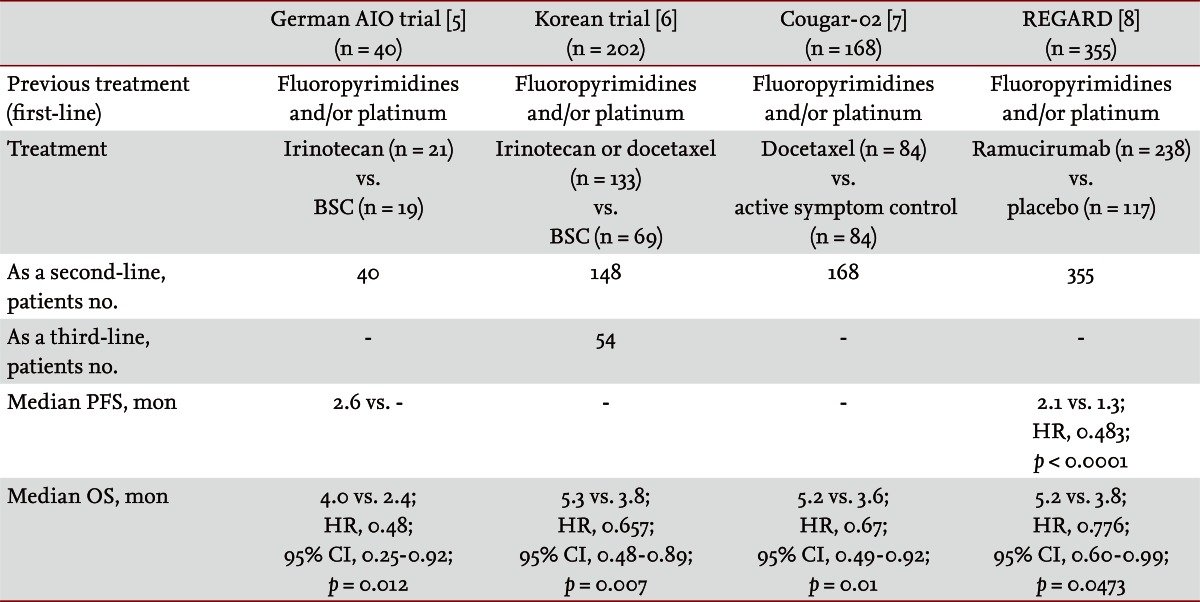
Many clinicians consider second-line chemotherapy after failure of first-line chemotherapy for patients with advanced gastric carcinoma [3]. Phase II trials and retrospective analyses have provided evidence that second-line is effective [4]. Recently, randomized phase III trials have strongly indicated that second line or further chemotherapy is more advantageous than supportive care (Table 1). A German trial found that irinotecan monotherapy improved overall survival compared to best supportive care [5]. A Korean trial found that irinotecan or docetaxel monotherapy prolonged overall survival compared to best supportive care and there was no difference in the treatment effect of docetaxel and irinotecan (p = 0.116) [6]. In the 2013 the American Society of Clinical Oncology (ASCO) Gastrointestinal Cancer Symposium, it was reported that docetaxel [7] and ramucirumab [8] demonstrated clinical benefit over supportive care in two phase III trials. Both agents significantly prolonged overall survival (Table 1). There is ample evidence to support the use of second line treatment in advanced gastric cancer. However, the issue of which regimen is a standard second-line treatment has not been clarified.
Little information concerning the survival advantage of third-line chemotherapy is extant. In a Korean phase III trial, the survival benefit in the chemotherapy arm was preserved in the further chemotherapy group (hazard ratio, 0.812; 95% confidence interval [CI], 0.450 to 1.464) (Table 1). Several retrospective studies presented the natural history of advanced gastric cancer with sequential salvage chemotherapy following first-line treatment [4]. The survival prolongation by second- and third-line salvage chemotherapy indicates its feasibility in selected patients.
After failure of first-line chemotherapy based on platinum and fluoropyrimidine, irinotecan, or taxane-based regimens have benefited survival and led to the same clinical outcome as salvage chemotherapy in advanced gastric cancer patients [4,6]. Based on the lack of cross-resistance between irinotecan and taxane, both regimens are plausible salvage treatment options. Additionally, it is necessary to select patients for salvage chemotherapy based on survival predictors including performance status, chemotherapy-free interval, response duration, metastatic pattern, tumor burden, and serum carcinoembryonic antigen level [4,6].
Lee and colleagues [9] evaluated the efficacy and toxicity of docetaxel monotherapy, 75 mg/m2 on day 1 every 3 weeks, in advanced gastric cancer patients who did not respond to oxaliplatin with leucovorin and 5-fluorouracil (m-FOLFOX-4), or to irinotecan with leucovorin and 5-fluorouracil (m-FOLFIRI). This retrospective study included thirty three patients and reported an overall response of 15%, time to progression of 2.1 months (95% CI, 1.63 to 2.58), and an overall survival time of 4.7 months (95% CI, 3.20 to 6.20). The results are comparable to previous reports of the efficacy of third-line treatment. This study provides important evidence that docetaxel is a feasible third-line therapy regimen after m-FOLFIRI and m-FOLFOX-4 regimens. A randomized prospective trial could further support this conclusion.
Trastuzumab, a molecular target agent, was approved for HER2 amplified gastric cancer patients, and other anti-HER2 agents-including lapatinib-have been evaluated as first- or second-line treatments. Furthermore, studies have been performed to elucidate biomarkers of chemotherapeutic agents and to investigate molecular biological features, including genetic and epigenetic profiles [10]. On the basis of those outcomes, randomized trials of target agents, and molecular biologic markers would facilitate treatment tailored to the individual patient.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





