To the Editor,
Macrolides have been proposed as potential antineoplastic and immunomodulatory agents [1]. Macrolides have anti-lymphoproliferative effects and are clinically applicable in lymphoid malignancies. Several previous reports have indicated that malignant lymphoma can be treated successfully using clarithromycin (CAM) [1,2]. We have previously reported two cases of malignant lymphoma successfully treated with CAM alone or CAM+prednisolone (PSL) (CamP) [3,4]. Here, we report a case of recurrent follicular lymphoma (FL) treated successfully with CAM, PSL, and cyclophosphamide (CPM) (CamP-C).
A 60-year-old male patient suffering from FL attended our hospital for the first time in 2 years with severe edema of the lower extremities, low fever, and thrombocytopenia, as identified at another clinic. At 49 years old, he had been diagnosed with stage III FL, grade 1, based on biopsy specimens of lymph nodes showing well-circumscribed follicles, with a monotonous population of predominantly small-cleaved cells that were positive for CD20, biopsy specimens of bone marrow, computed tomography (CT), and endoscopies.
He initially received six cycles of CHOP chemotherapy (CPM, adriamycin, vincristine, and PSL) and achieved complete remission (CR). Recurrent FL was recognized at 53 years old, at which time he was treated with six cycles of rituximab (R)-CHOP chemotherapy and two subsequent cycles of R monotherapy. CR was again apparently achieved. Recurrent FL, the extent of which was slight, was again recognized at 58 years old. Because he was not experiencing any symptom related to recurrent FL, he stopped visiting our hospital.
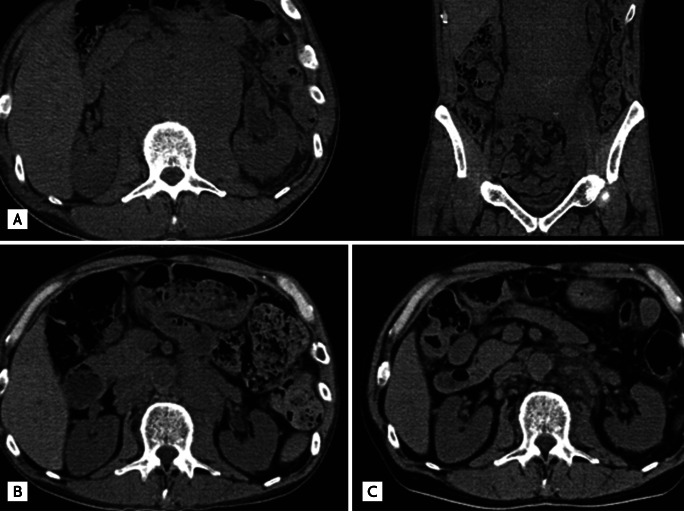
On this visit, for the first time in 2 years, CT showed severe para-aortic lymphadenopathy, hepatomegaly, and left ureteral dilatation (Fig. 1A). Because the edema was attributed to para-aortic lymphadenopathy and there was considered to be a risk of hydronephrosis in the left kidney, we decided to initiate chemotherapy. However, thrombocytopenia, due to myelodysplastic syndrome, likely resulting from the prior chemotherapy described above, was evident and his platelet count was ~50 ├Ś 109/L.
Consequently, we decided to begin treatment with CamP-C as an alternative to conventional chemotherapy, after obtaining informed consent. The patient received CAM 400 mg, twice daily, over the long-term, PSL 10 mg, three times daily, for 1 week, then 10 mg, twice daily, for 1 week, then 5 mg, three times daily, for 2 weeks, and finally 5 mg, twice daily, for the long-term, and CPM 50 mg, once daily, for the long-term.
At 4 months after starting this treatment, CT showed marked improvement of the para-aortic lymphadenopathy, hepatomegaly, and left ureteral dilatation (Fig. 1B), with gradual improvement of the edema. At the same time, soluble interleukin-2 receptor (sIL-2R) levels, which are closely related to disease activity and the response to chemotherapy, decreased, from 4,627 to 1,860 U/mL.
The patient suffered from acute pneumonia twice and platelet counts decreased to ~30 ├Ś 109/L at times during this treatment, so, after obtaining informed consent, he was treated with CamP, comprising CAM 400 mg, twice daily, over the long-term and PSL 5 mg, twice daily, for the long-term, to prevent severe thrombocytopenia and infections. At 8 months after starting administration of CamP, para-aortic lymphadenopathy, hepatomegaly, and left ureteral dilatation improved further and sIL-2 levels decreased, to 616 U/mL (Fig. 1C).
We previously reported a case of bcl-2-positive diffuse large B-cell lymphoma (DLBCL) that was treated successfully with CamP [4]. Because CAM is known to induce down-regulation of bcl-2 protein in lymphoid cells [5] and glucocorticoids are known to induce apoptosis of lymphoid cells, we suggest that the CamP treatment had induced apoptosis of lymphoma cells in our previous case. Because no superficial lymph nodes, other than the para-aortic lymph nodes, were palpable and the platelet count was 52 ├Ś 109/L in this recurrence, we did not rebiopsy the lymph nodes.
Whether this case transformed into high-grade lymphoma, such as DLBCL, remains unclear. However, the initial chromosome analysis of the lymph nodes identified t (14;18)(q32;q21) and an immunohistochemical study for bcl-2 demonstrated positive staining in lymphoma cells in this case, so CamP-C treatment and CamP treatment were considered likely to prove effective via apoptosis of lymphoma cells, as in our previous case. Because edema was severe and hydronephrosis might have occurred sooner or later, the patient was treated initially and successfully with CamP-C.
After experiencing several episodes of pneumonia and severe thrombocytopenia during CamP-C treatment, the patient was treated successfully with CamP, with the intention of preventing severe thrombocytopenia and infections. Given that CamP-C treatment improved abdominal lymphadenopathy and sIL-2R levels more rapidly than CamP treatment, CamP-C treatment was considered more effective. However, because platelet counts did not decrease markedly and he did not suffer from infections after CamP treatment, the present case suggests that CamP treatment may be safer than CamP-C treatment.
Although our patient appears to have benefited from CamP-C and CamP treatments, those regimens should not be considered recommended therapies for bcl-2-positive lymphoma patients who can receive conventional chemotherapies. The present case demonstrates that these treatments may be options for those bcl-2-positive lymphoma patients, including recurrent/refractory lymphoma patients, who cannot receive conventional chemotherapies due to underlying factors, such as advanced age, poor performance status, cytopenia, and susceptibility to infection. Although our experience is limited to only two cases, we tentatively recommend CamP-C and CamP as treatment options for such patients.
Regarding specific details of the regimens, the CAM dosage was 400 mg, twice daily, for the long-term. The PSL dosage was initially 0.5 mg/kg, and was reduced gradually to 10 mg/day over the course of 1 month. For example, if the initial PSL dosage is 20 mg/day, 10 mg is given, twice daily, for 2 weeks, reducing to 5 mg, three times daily, for 2 weeks, and then 5 mg, twice daily, for the long-term. Whatever the initial dosage, the ultimate PSL dosage is 10 mg/day. In CamP-C treatment, the CPM dosage was 50 mg, once daily, for the long-term. If long-term use of CAM causes arrhythmia as a side effect, CAM administration should be stopped. Similarly, if long-term use of CPM causes severe cytopenia, CPM administration should be stopped. More research is required to determine optimal doses and durations, and to assess response rates and tolerability, before these treatments can be recommended on a wider basis.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





