 |
 |
| Korean J Intern Med > Volume 28(6); 2013 > Article |
|
Abstract
Background/Aims
Endoscopic submucosal dissection (ESD) has become accepted as a minimally invasive treatment for gastric neoplasms. However, the development of synchronous or metachronous gastric lesions after endoscopic resection has become a major problem. We investigated the characteristics of multiple gastric neoplasms in patients with early gastric cancer (EGC) or gastric adenoma after ESD.
Methods
In total, 512 patients with EGC or gastric adenoma who had undergone ESD between January 2008 and December 2011 participated in this study. The incidence of and factors associated with synchronous and metachronous gastric tumors were investigated in this retrospective study.
Results
In total, 66 patients (12.9%) had synchronous lesions, and 13 patients (2.5%) had metachronous lesions. Older (> 65 years) subjects had an increased risk of multiple gastric neoplasms (p = 0.012). About two-thirds of the multiple lesions were similar in macroscopic and histological type to the primary lesions. The median interval from the initial lesions to the diagnosis of metachronous lesions was 31 months. The annual incidence rate of metachronous lesions was approximately 3%.
Conclusions
We recommend careful follow-up in patients of advanced age (> 65 years) after initial ESD because multiple lesions could be detected in the remnant stomach. Annual surveillance might aid in the detection of metachronous lesions. Large-scale, multicenter, and longer prospective studies of appropriate surveillance programs are needed.
Endoscopic submucosal dissection (ESD) has become a useful method for the treatment of selected cases of gastric neoplasms, enabling en bloc resection [1-5]. ESD is an effective and minimally invasive procedure, and it can preserve more of the stomach than surgical resection. However, the remnant stomach after such treatment is at high risk for the development of multiple gastric lesions, defined as neoplasms that develop subsequently at another site in the stomach [6-8]. In fact, the reported incidence of cancer in the gastric remnant is 1.8% to 5% in patients who have undergone surgical treatment for gastric cancer [9,10], while the reported incidence of multiple gastric cancers is 9% to 24% in patients who have undergone endoscopic resection of a gastric cancer [6,11-13].
Despite numerous reports on the characteristics of synchronous and metachronous lesions after endoscopic treatment [6,7,11-14], appropriate surveillance strategies after gastric endoscopic treatment remain unclear. Here, we describe the incidence of and factors associated with synchronous and metachronous gastric lesions in patients with early gastric cancer (EGC) or adenoma after ESD.
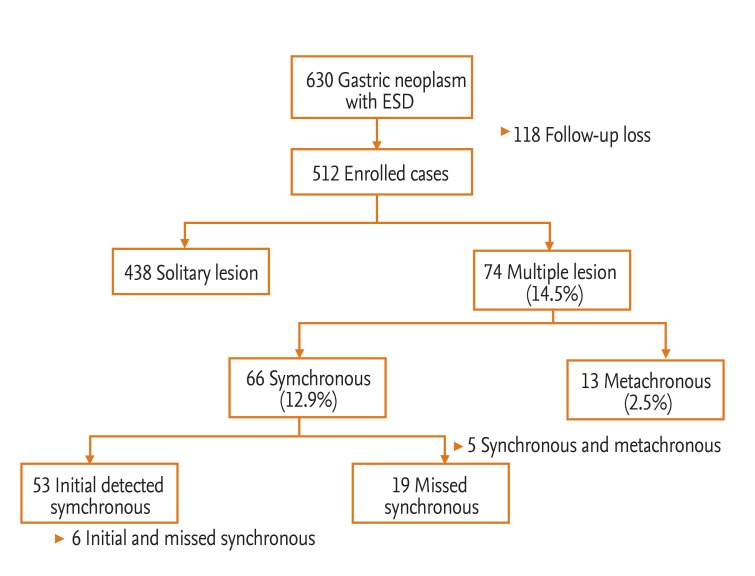
From January 2008 to December 2011, 630 patients with EGC or adenoma were treated with ESD at Presbyterian Medical Center (Jeonju, Korea). Of the 630 patients, we excluded 118 who were followed for < 1 month; thus, 512 patients who underwent ESD for EGC or adenoma were reviewed retrospectively (Fig. 1). Each ESD had been undertaken with curative intent and informed written consent was obtained from all patients before the procedure. Initial and periodic endoscopic examinations were performed at Presbyterian Medical Center. Follow-up esophagogastroduodenoscopy (EGD) was started within 3 months after the initial ESD; surveillance EGD was performed every 6 to 12 months thereafter.
Clinical characteristics, histopathology, and tumor recurrence were analyzed. All endoscopic findings were reviewed for data related to the size, location, and gross findings of the tumor. We compared the clinicopathological factors between two patient groups: solitary versus multiple lesions. For patients with multiple lesions, only the main lesions were compared. In cases with missed synchronous and metachronous lesions, the initial ESD lesion was considered the main lesion. For patients with initial synchronous lesions, the most malignant or dysplastic lesion was considered the main lesion. In the case of initially detected multiple lesions with the same histology, the largest lesion was considered the main lesion. Tumor location (long axis) was classified by dividing the stomach into three equal sections: upper (cardia, fundus, and upper body), middle (midbody, lower body, and angle), and lower (antrum and prepylorus). The maximum tumor diameter in the gastric lesion was measured as the tumor size. The gastric lesions were divided into two gross types: elevated and depressed lesions. Information regarding the status of the background gastric mucosa and intestinal metaplasia was evaluated histologically in 435 patients. We analyzed the incidence of synchronous and metachronous lesions and yearly incidence rates of metachronous lesions after ESD. Furthermore, we compared the clinicopathological factors between primary and secondary lesions in patients with multiple lesions.
We defined lesions that developed subsequently at another site in the stomach after ESD as multiple gastric neoplasms, according to Moertel's criteria [15]. We considered gastric neoplasms detected within 1 year after ESD as synchronous lesions. These consisted of initially detected multiple neoplasms and missed synchronous lesions, defined as secondary gastric neoplasms detected within 1 year of ESD but missed endoscopically at the time of ESD [14]. Metachronous lesions were defined as secondary gastric neoplasms occurring at least 1 year after the initial ESD [6-8,11,12].
We performed chi-square and Mann-Whitney U tests to compare the clinicopathological factors between the groups. Factors affecting multiple gastric neoplasms were identified using a multivariate logistic regression with SPSS software version 19 (IBM Co., Armonk, NY, USA); p < 0.05 was considered to indicate statistical significance.
Of 630 EGC or adenoma patients who were initially treated with ESD, 512 were followed by endoscopy and were included in the analysis. The characteristics of these 512 patients are listed in Table 1. The median age at diagnosis was 66 years (range, 23 to 90), and the mean follow-up period by endoscopy was 16 months (range, 1 to 52). No patient died of a gastric neoplasm, and there were no treatment-related deaths.
In total, 66 patients (12.9%) developed synchronous gastric neoplasms, of which 53 had initially detected synchronous lesions and 19 had missed synchronous lesions; six patients had both initial synchronous and missed synchronous lesions. A total of 13 patients (2.5%) developed metachronous gastric neoplasms and 74 (14.5%) had synchronous and/or metachronous multiple gastric lesions (Fig. 1). Five patients had both synchronous and metachronous lesions. Ten patients had more than three gastric neoplasms; one of them had multiple gastric neoplasms initially, and nine patients subsequently had third or fourth gastric neoplasms. The median interval between the primary ESD and diagnosis of the first metachronous neoplasm was 31 months (range, 15 to 45). One patient with metachronous EGC underwent surgery because of a combined cholangiocarcinoma. The remaining 12 patients were successfully treated with endoscopic resection. The mean annual incidence rate of metachronous lesions was approximately 3% (Fig. 2).
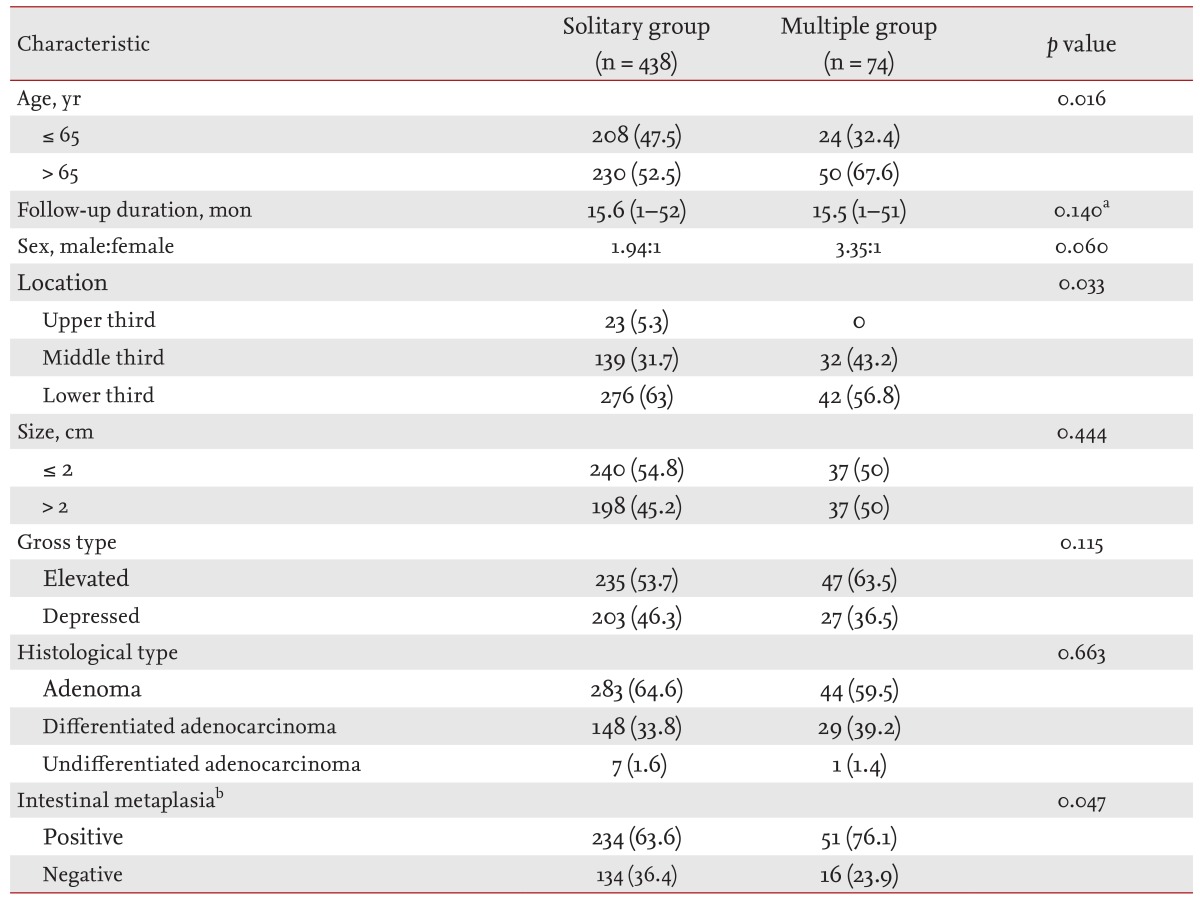
In a univariate analysis, multiple lesions occurred more frequently in patients of advanced age (> 65 years), and in the middle-third location (p = 0.016 and p = 0.033, respectively). Histopathological evaluations were performed on 435 patients (solitary group, n = 368; multiple group, n = 67) during the 12 months before and after ESD. The presence of intestinal metaplasia in the surrounding mucosa correlated significantly with multiple lesions (p = 0.047) (Table 1).
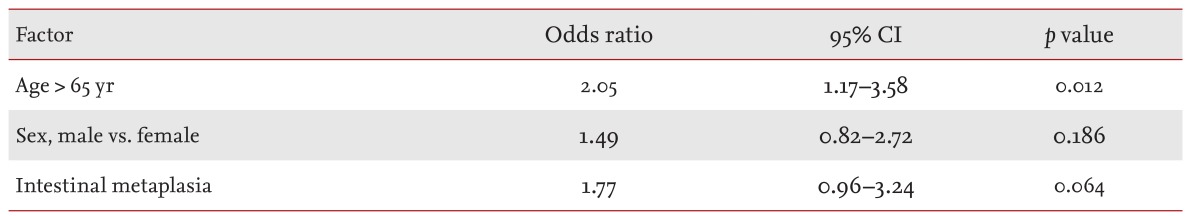
In a multivariate analysis of the occurrence of the multiple lesions, only old age (> 65 years) showed a significant correlation (odds ratio, 2.05; 95% confidence interval, 1.1 to 3.5; p = 0.012). Intestinal metaplasia and sex showed no association with multiple lesions (Table 2).
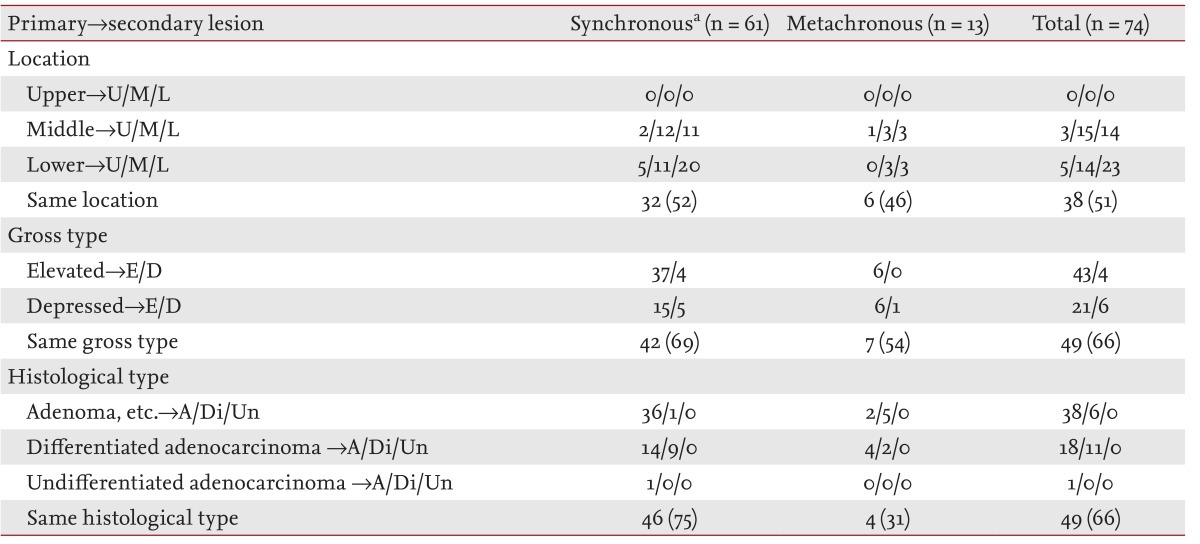
The clinicopathological characteristics of the 74 patients who had multiple gastric lesions are shown in Table 3. Half of these lesions were located in the same third of the stomach as the primary lesions, and two-thirds of these lesions showed similarities in macroscopic and histological type with the primary lesions.
Endoscopic resection is now widely accepted as a standard treatment for selected cases of gastric neoplasms [2-4,8]. While endoscopic resection can preserve the stomach and help maintain the postoperative quality of life for patients, endoscopic resection increases the risk of synchronous or metachronous multiple lesions in the remnant gastric mucosa [6-8,11-13]. Thus, establishing an optimal surveillance strategy is important for detecting multiple lesions during the early stages of disease and avoiding surgery for secondary lesions [7,8].
There are several reports on the predictive factors for multiple lesions after endoscopic resection. Arima et al. [6] reported that metachronous recurrence was more frequent in patients with synchronous multiplicity, while Han et al. [11] reported that old age and antrum atrophy were associated with metachronous cancer. Seo et al. [13] also noted that an undifferentiated histology of the EGC may predict multiple lesions. In this study, only patients of advanced age (> 65 years) showed an increased risk of multiple gastric neoplasms. Several studies have shown a significant effect of Helicobacter pylori eradication on reducing the incidence of metachronous cancer after endoscopic resection for EGC [16,17]. Additionally, it was reported that patients with microsatellite instability-positive and intestinal claudin-positive cancers had a significantly higher risk of both synchronous and metachronous multiple cancers after endoscopic resection [18-21].
Previous studies have shown that the mean annual incidence rate of metachronous lesions after endoscopic resection was 3% to 4%, while the median interval between primary endoscopic resection and the diagnosis of a metachronous lesion was 30 to 37 months [7,8,11,12]. Consistent with these data, in our study, the mean annual incidence rate of metachronous lesions was 3%, and the median interval of occurrence of metachronous lesions was 31 months.
To date, the optimal surveillance schedule for detecting multiple lesions remains unclear. In this study, follow-up EGD was started within 3 months after the initial ESD, and surveillance EGD was performed every 6 to 12 months. Those patients with metachronous lesions, except one patient with EGC and cholangiocarcinoma, were treated successfully with endoscopic resection. In one clinical study of metachronous gastric neoplasms, the cumulative incidence curve of metachronous occurrence generally increased steadily to 6.5 years and was generally flat to 10 years [7].
There are four reports on metachronous cancer evaluated with more than 10 years of follow-up after endoscopic resection; in those reports, no metachronous cancer was detected after a 10-year interval [7,12,22,23]. In particular, Kobayashi et al. [23] reported that the high risk of metachronous cancers did not continue after 10 years. Based on these studies, we suggest that annual surveillance is needed for at least 5 years and probably until 10 years after the initial ESD.
In a study by Nasu et al. [12], half of the multiple lesions were located in the same third of the stomach, and most multiple lesions were similar in macroscopic and histological type to the primary lesions. We found similar results in this study. Therefore, similarities in multiple lesions may aid the detection of secondary lesions during follow-up endoscopy.
Our study has several limitations. First, the mean observation period was relatively short at 16 months. Second, this study had insufficient exclusion criteria; patients with additional gastrectomies, incomplete resection (piecemeal, margin-positive, or unclear), and follow-up periods of < 1 year were included in our population. Third, our data on the presence of intestinal metaplasia were incomplete because they were collected retrospectively from medical records.
In conclusion, we recommend more careful follow-up in patients of advanced age (> 65 years) after gastric ESD, and endoscopists should make note of similarities among multiple lesions. Annual surveillance might help in detecting metachronous lesions after an initial ESD. Large-scale, multicenter, and longer prospective studies are needed to determine the most appropriate surveillance strategy.
1. Older (> 65 years) patients with early gastric cancer or gastric adenoma who had undergone endoscopic submucosal dissection had an increased risk of multiple gastric neoplasms.
2. About two-thirds of the multiple lesions were similar in macroscopic and histological type to the primary lesions.
3. Annual surveillance might aid in the detection of metachronous lesions.
References
1. Lee H, Yun WK, Min BH, et al. A feasibility study on the expanded indication for endoscopic submucosal dissection of early gastric cancer. Surg Endosc 2011;25:1985ŌĆō1993PMID : 21136092.


2. Isomoto H, Shikuwa S, Yamaguchi N, et al. Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut 2009;58:331ŌĆō336PMID : 19001058.


3. Goto O, Fujishiro M, Kodashima S, Ono S, Omata M. Outcomes of endoscopic submucosal dissection for early gastric cancer with special reference to validation for curability criteria. Endoscopy 2009;41:118ŌĆō122PMID : 19214889.


4. Kato M, Nishida T, Tsutsui S, et al. Endoscopic submucosal dissection as a treatment for gastric noninvasive neoplasia: a multicenter study by Osaka University ESD Study Group. J Gastroenterol 2011;46:325ŌĆō331PMID : 21107615.


5. Chung IK, Lee JH, Lee SH, et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc 2009;69:1228ŌĆō1235PMID : 19249769.


6. Arima N, Adachi K, Katsube T, et al. Predictive factors for metachronous recurrence of early gastric cancer after endoscopic treatment. J Clin Gastroenterol 1999;29:44ŌĆō47PMID : 10405230.


7. Nakajima T, Oda I, Gotoda T, et al. Metachronous gastric cancers after endoscopic resection: how effective is annual endoscopic surveillance? Gastric Cancer 2006;9:93ŌĆō98PMID : 16767364.


8. Kato M, Nishida T, Yamamoto K, et al. Scheduled endoscopic surveillance controls secondary cancer after curative endoscopic resection for early gastric cancer: a multicentre retrospective cohort study by Osaka University ESD study group. Gut 2012;8. 21. [Epub]. 10.1136/gutjnl-2011-301647.

9. Takeda J, Toyonaga A, Koufuji K, et al. Early gastric cancer in the remnant stomach. Hepatogastroenterology 1998;45:1907ŌĆō1911PMID : 9840174.

10. Nicholls JC. Stump cancer following gastric surgery. World J Surg 1979;3:731ŌĆō736PMID : 532192.


11. Han JS, Jang JS, Choi SR, et al. A study of metachronous cancer after endoscopic resection of early gastric cancer. Scand J Gastroenterol 2011;46:1099ŌĆō1104PMID : 21668406.


12. Nasu J, Doi T, Endo H, Nishina T, Hirasaki S, Hyodo I. Characteristics of metachronous multiple early gastric cancers after endoscopic mucosal resection. Endoscopy 2005;37:990ŌĆō993PMID : 16189772.


13. Seo JH, Park JC, Kim YJ, Shin SK, Lee YC, Lee SK. Undifferentiated histology after endoscopic resection may predict synchronous and metachronous occurrence of early gastric cancer. Digestion 2010;81:35ŌĆō42PMID : 20029207.


14. Yoo JH, Shin SJ, Lee KM, et al. How can we predict the presence of missed synchronous lesions after endoscopic submucosal dissection for early gastric cancers or gastric adenomas? J Clin Gastroenterol 2013;47:e17ŌĆōe22PMID : 22810109.


15. Moertel CG, Bargen JA, Soule EH. Multiple gastric cancers: review of the literature and study of 42 cases. Gastroenterology 1957;32:1095ŌĆō1103PMID : 13438166.


16. Fukase K, Kato M, Kikuchi S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet 2008;372:392ŌĆō397PMID : 18675689.


17. Uemura N, Mukai T, Okamoto S, et al. Effect of Helicobacter pylori eradication on subsequent development of cancer after endoscopic resection of early gastric cancer. Cancer Epidemiol Biomarkers Prev 1997;6:639ŌĆō642PMID : 9264278.


18. Miyoshi E, Haruma K, Hiyama T, et al. Microsatellite instability is a genetic marker for the development of multiple gastric cancers. Int J Cancer 2001;95:350ŌĆō353PMID : 11668515.


19. Kawamura A, Adachi K, Ishihara S, et al. Correlation between microsatellite instability and metachronous disease recurrence after endoscopic mucosal resection in patients with early stage gastric carcinoma. Cancer 2001;91:339ŌĆō345PMID : 11180080.


20. Hasuo T, Semba S, Li D, et al. Assessment of microsatellite instability status for the prediction of metachronous recurrence after initial endoscopic submucosal dissection for early gastric cancer. Br J Cancer 2007;96:89ŌĆō94PMID : 17179982.



21. Semba S, Hasuo T, Satake S, Nakayama F, Yokozaki H. Prognostic significance of intestinal claudins in high-risk synchronous and metachronous multiple gastric epithelial neoplasias after initial endoscopic submucosal dissection. Pathol Int 2008;58:371ŌĆō377PMID : 18477216.


22. Tada M, Higaki S, Matsumoto Y, Ryo S, Karita M. Strip biopsy: its problems and measures implied by a long-term follow-up study (simultaneous and metachronous multiple cancers). Stomach Intest 1993;28:1441ŌĆō1451.
Figure┬Ā2
Annual incidence rates of metachronous lesions after primary endoscopic submucosal dissection (ESD).

Table┬Ā1
Baseline characteristics of those patients with gastric neoplasms and their initially resected lesions
Table┬Ā2
Predictive factors associated with multiple lesions based on a multivariate logistic regression analysis



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



