INTRODUCTION
In Japan, approximately 140,000 patients with ulcerative colitis (UC) and 40,000 with Crohn disease (CD) are currently registered by the Japanese Health, Labor and Welfare Ministry [1]. Because health-care costs for inflammatory bowel diseases (IBDs), including UC and CD, of registered patients are generally covered by the government, most patients voluntarily join the registry. Registration, however, is not mandatory, and some patients with mild to moderate IBD may refuse to register because of privacy concerns. Therefore, the actual numbers of IBD patients in Japan may be 20% to 40% higher than the numbers in the registry. The incidence of IBD in Japan ranks it as a low- to moderate-frequency country [2,3,4], although the incidence and prevalence is rapidly increasing [1].
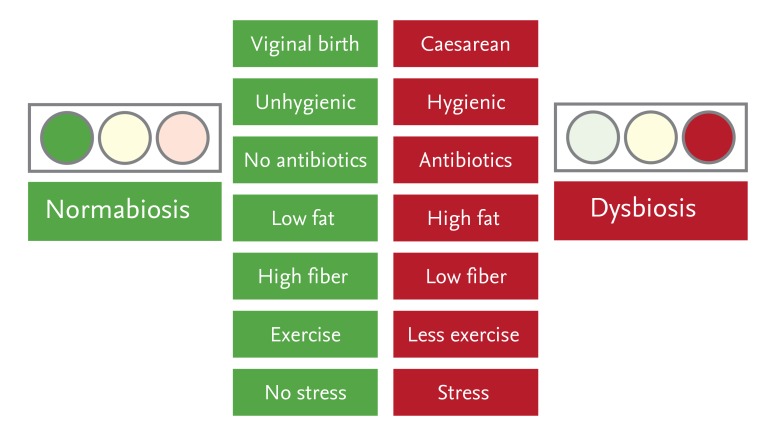
Advances in next-generation gene sequencing technology have resulted in the identification of over 160 IBD-associated susceptibility genes within the past 10 years [5]. However, these susceptibility genes are unlikely to be the primary cause of IBD in Asia, because in the past 30 years the numbers of IBD patients in Japan have increased 100-fold. It is more likely that dramatic changes in the Japanese social environment, especially dietary habits that lead to an unhealthy composition of microbiota, known as dysbiosis, are fundamental causes of IBD [6,7,8,9,10,11,12,13,14,15]. Japan now has an upgraded water supply and sewerage systems along with dietary habits and overuse of antibiotics [16] that are similar to those found in developed Western countries (Fig. 1). Indeed, residents of Tokyo can live in an environment with exactly the same food and hygienic conditions as in New York.
RAPID DIETARY HABIT CHANGES IN JAPAN
Until about 150 years ago, Japan was officially sealed off from the outside world. Most Japanese individuals had no contact with Western people or Western dietary habits, and ate traditional Japanese foods. After the end of the Edo era in 1868, the new Japanese government opened the country to Westerners and began diplomatic and cultural contract with many Western countries. Concurrently, the Japanese government promoted a Western lifestyle, including Western diets, housing, clothes, and culture. However, only a small proportion of Japanese people, known as the favored classes, could afford Western foods, while the vast majority continued to eat frugal Japanese foods for an additional 100 years. A typical Japanese diet at that time was a simple vegetarian meal composed of unthreshed rice mixed with barley, miso soup with root vegetables and/or tofu, small grilled fermented fish, and fermented pickled vegetables. Fermentation was essential to preserve foods in the absence of cooling systems. After the end of World War II in 1945, democracy emerged in Japan, with many people choosing Westernization. Annual reports by the Japanese Health, Labor and Welfare Ministry have shown rapid increased intake of sugar-rich carbonated beverages, fat- and carbohydrate-rich Western snacks (e.g., potato chips), and animal protein and fat, and a concurrent rapid decrease in the intake of dietary fiber.
CORRELATION BETWEEN DIET AND MICROBIOTA
The microbiota/microbiome field has been described in many recent review articles [17,18,19,20,21,22,23]. The availability of next-generation sequencing has had the greatest impact on this field, as it can be used to sequence numerous bacterial DNA sequences simultaneously using a shot-gun method [24,25,26,27,28,29]. Each human being contains 100 trillion bacteria, composed of over 200 species with 50-fold more genes that in the human genome [28,29]. Dysbiosis and loss of microbiome diversity is thought to result in many kinds of diseases and predisease conditions. These include not only intestinal immune diseases (e.g., IBD) [9,10,30] and functional diseases (e.g., irritable bowel syndrome) [31,32] but also extraintestinal diseases such as obesity, arteriosclerosis, allergy, and autism disorders [33,34,35,36,37]. The incidence of all these diseases is increasing in developed Western and Asian countries, irrespective of whether they are T helper (Th) 1- or Th2-mediated diseases.
The organisms constituting a healthy composition of microbiota, or normabiosis, remain unclear for humans or animals. Moreover, it is unclear whether normabiosis is similar in healthy individuals and between Western and Asian people. However, striking differences in the composition of microbiota have been observed, not only between diseased (e.g., IBD) and healthy individuals but also between different diseased individuals. For example, the composition of microbiota in healthy African children from Burkina Faso, a country with a low incidence of IBD, included greater amounts of Prevotella, greater microbial diversity, and higher levels of short chain fatty acids (SCFA) than the microbiota of healthy European children from Italy, a country with a high incidence rate of IBD [38]. Similar results were observed when the microbiota of healthy individuals from South America and South Asia were compared with the microbiota of healthy individuals from an industrialized country such as the United States [39].
POOR EVIDENCE OF SPECIFIC DIETARY COMPONENTS IN THE ETIOLOGY OF IBD
Dramatic changes in dietary components, including increased sugar/refined carbohydrates and animal fat/protein and reductions in dietary fibers (prebiotics, fermentable oligosaccharides), fruits/vegetables, and fermented products containing probiotics, have been proposed as major etiologic factors in the development of both UC and CD [40,41]. Additionally, the hygienic environment in industrial countries may be closely associated with a lower likelihood of coming in contact with fermented bacteria, which may be identical to probiotics [42]. Surprisingly, however, there is little evidence showing that specific dietary components are risk factors for the development of UC and CD [43,44,45]. However, it may be difficult or impossible to determine the real causes of IBD, because some individuals may consume both Western snacks and Japanese foods. Nevertheless, many researchers and clinicians strongly believe that current dietary habits, of high fat/low fiber and less fiber/probiotics, may be improved by returning to diets consumed during the era before modernization.
SOLID EVIDENCE OF SPECIFIC DIETARY COMPONENTS IN THE ETIOLOGY OF IBD IN ANIMAL MODELS
Results from animal disease models provide clearer evidence of the involvement of specific dietary components in the etiology of IBD [46,47]. However, a direct translation of animal results to human diseases is problematic. For example, mice without colitis cohoused with colitic mice developed similar colitis with a shift to dysbiosis [48], while germ-free mice transplanted with feces of obese mice became obese and those transplanted with feces of lean mice became lean [49].
Several hypotheses have been proposed to explain the critical roles of probiotic microbiota in the prevention of IBD in mouse models. This may be shown using a gnotobiotic system, in which germ-free mice are inoculated with one or several specific strains of bacteria, allowing the specific roles of these bacteria to be evaluated in vivo (Fig. 2). Germ-free mice inoculated with a mixture of 46 mouse-derived Clostridium coccoides and Clostridium leptum strains had a normal proportion of mucosal interleukin (IL)-10-producing regulatory T-cells, equivalent to those in specific pathogen-free normal mice [50]. These T-cells stimulated the production of transforming growth factor-b from colonic epithelial cells, resulting in resistance to experimental colitis [50]. Furthermore, C. coccoides and C. leptum from healthy human volunteers were similarly able to induce regulatory T-cells in mice [51]. Additionally, fermented Clostridia probiotics locally produced SCFAs, including butyrate, through the fermentation process, and these SCFA directly induced IL-10-producing regulatory T-cells [52,53,54]. These results indicate that probiotic-induced SCFAs are beneficial in maintaining colonic epithelial cells and in providing energy for hepatocytes. In contrast to the induction of regulatory T-cells by probiotics, we recently proposed a distinctive mechanism of probiotic actions, based on findings that a probiotic strain, Clostridium butyricum, which preferentially produces butyrate, suppressed the development of acute experimental colitis in mice by inducing IL-10-producing mucosal macrophages in a toll-like receptor-2/MyD88-dependent manner [55].
The mechanism by which a high-fat diet is associated with IBD onset remains unknown. The incidence of colitis was markedly increased in milk fat-fed IL-10-deficient mice, but not in normal mice or polyunsaturated fat-fed IL-10-deficient mice, with Bilophila wadsworthi observed in the feces of milk fat-fed mice, indicating dysbiosis [56]. This finding was clearly linked with taurine conjugation of hepatic bile acids by B. wadsworthi, with direct evidence showing that IL-10-deficient mice fed a low-fat diet containing taurocholic acid, but not glycocholic acid, developed colitis. This paper was the first demonstration that a specific Western-style diet containing high amounts of certain saturated fats enhanced the development of colitis in mice via a specific molecule, taurocholic acid.
JAPANESE AND KOREAN FOODS ARE RECOMMENDED FOR IBD PATIENTS
Prebiotic evidence
Despite findings in many animal models of colitis, decreased dietary fiber intake is not definitely associated with IBD development. However, in daily clinical practice, some dieticians may instruct IBD patients to avoid dietary fiber, at least during the inflammatory stages, because fiber may stimulate the intestinal mucosa. Patients with CD and severe stenosis of the intestine may also be advised to avoid dietary fiber because of the possible intestinal obstruction. However, in our clinical practice at Keio University Hospital, which treats > 2,000 IBD patients, we have explained recently published findings on the roles of dietary fiber in the suppression of inflammation to patients, and have recommended that these patients consume more dietary fiber, including fruits, vegetables, seaweeds, dried mushrooms and dried Japanese radishes. Modern Japanese individuals prefer hulled white rice to unthreshed brownish rice, which contains rice bran with a glucan fiber-rich component. Additionally, wheat bran is discarded when making soft white bread. Because the modern Japanese lifestyle may not allow individuals to eat proper meals, we recommend that IBD patients consume boiled rice together with an individually acceptable proportion of barley, which was a historical part of the normal Japanese lifestyle. The goal is to gradually increase the intake of properly balanced soluble and insoluble dietary fiber every month or year, because both the proportion and absolute numbers of fermented probiotics strains are reduced in the intestines of IBD patients [57]. A gradual increase in fermenters may allow patients to handle increased amounts of dietary fiber. The quantity of fermenters present in the intestines of these patients at the beginning of probiotic treatment may be insufficient to handle an overabundance of dietary fiber, resulting in deleterious outcomes, such as intestinal obstruction.
Probiotic evidence
The intake of foods containing fermented probiotics has decreased in Japan. Traditionally, Japanese have eaten fermented foods, such as fermented pickled vegetables, fermented bean paste, fermented stockfishes, fermented fish sushi, and natto. Fermentation was originally used to preserve foods and protect against putrefactive bacteria, such as pathobionts, prior to the widespread availability of electric refrigerators around 1963. Modern Japanese may avoid eating fermented foods owing to their strong smell. Indeed, modern Japanese, especially younger individuals, prefer light Kimchi, which can be made overnight, rather than sour Kimchi, which requires several months to ferment and produce abundant probiotics (Lactobacillus) and SCFAs. Additionally, young Japanese individuals do not like to eat Kusaya, a type of deeply fermented fish similar to Hongeohoe in Korea, both of which have powerful smells.
CONCLUSIONS
Randomized controlled trials have shown evidence for the effectiveness of fecal microbiota transplantation (FMT) in patients with recurrent Clostridium difficile infection (CDI) [58], and several case studies have shown the benefit of FMT for patients with IBD [58,59,60]. FMT may normalize dysbiosis in patients with IBD, but the strategy used in patients with recurrent CDI may have to be modified for patients with IBD. Although the incidence of IBD in developed Asian countries is rapidly increasing, so is the incidence in developed Western countries. Asian societies are at a crossroads between a Western-style and a traditional high-fiber, low-fat, and fermenter-rich diet. Clinicians should encourage these traditional foods to promote public welfare.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





