 |
 |
| Korean J Intern Med > Volume 29(5); 2014 > Article |
|
Abstract
Background/Aims
The treatment for steroid-refractory acute graft versus host disease (GVHD) after allogeneic stem cell transplantation (allo-SCT) needs to be standardized. We report our clinical experience with etanercept for steroid-refractory acute GVHD.
Methods
Eighteen patients who underwent allo-SCT and presented with steroid-refractory acute GVHD at Ajou University Hospital were studied retrospectively. They were given 25 mg of etanercept subcutaneously twice weekly for 4 weeks. The clinical responses were evaluated with regard to the severity of acute GVHD.
Results
The median patient age was 43.5 years. Using nonparametric tests, etanercept had a down-grading effect on acute GVHD (p = 0.005), although no patient experienced complete remission. Partial responses were seen in 80%, 17%, and 57% of grade II to IV patients, respectively. Skin and gut GVHD were well controlled with etanercept, whereas hepatic GVHD was not. Four patients died of fatal infections. No factors affecting the clinical outcome of etanercept were identified.
Despite the advances in medications and techniques that support allogeneic hematopoietic stem cell transplantation (allo-SCT), acute graft versus host disease (GVHD) remains a major cause of morbidity and mortality, reducing the survival benefit of transplantation. Although prophylaxis strategies have lowered the prevalence of acute GVHD, 35% to 50% of transplant patients still develop from acute GVHD. Corticosteroids have been the standard initial treatment for grades II to IV acute GVHD, with 25% to 41% of complete response (CR) rates [1,2,3]. However, the prognosis of corticosteroid-refractory acute GVHD is dismal, with a mortality rate of approximately 70% [4]. Over the past decade, numerous drugs for managing acute GVHD have been tested. However, randomized phase III trials are necessary to document the clinical benefit of those drugs over corticosteroids.
Two therapeutic approaches to steroid-refractory acute GVHD are available. One is based on cytotoxic agents that kill effector T cells, such as antithymocyte globulin, OKT3, or mycophenolate mofetil (MMF). The other is based on inhibiting the cytokines involved in the pathogenesis of acute GVHD, including antibodies against tumor necrosis factor α (TNF-α) and interleukin (IL)-2 or IL-1 antagonists [5,6]. Of these, TNF-α is a central cytokine in acute GVHD and is strongly implicated in its intestinal and cutaneous manifestations. This has led to studies of the use of antibodies against TNF in the prophylaxis and treatment of acute GVHD.
Etanercept is a fusion protein consisting of two identical chains of the human TNF receptor p75 monomer fused with the Fc domain of human IgG1. It binds to soluble TNF-α, neutralizing its activity. Preclinical data indicate that etanercept blocks lymphotoxin, formerly known as TNF-β, which destroys cells during acute GVHD via the TNF receptor pathway, without activating donor T cells [7]. Etanercept reduces the risk of infection compared to infliximab due to its minimal cytotoxic effect on phagocytic cells expressing surface TNF-α [8]. Therefore, there are many reports on the use of etanercept to treat acute GVHD.
We report our experience regarding the clinical efficacy and safety of etanercept, a recombinant human soluble tumor necrosis factor receptor fusion protein, in treating steroid-refractory acute GVHD after allo-SCT in patients with hematological diseases.
We reviewed the medical records of 18 consecutive adult allo-SCT patients who received etanercept (Enbrel, Pfizer, Seoul, Korea) to treat steroid-refractory acute GVHD. The clinical outcomes, including acute GVHD and underlying diseases, responsiveness to etanercept, overall survival after transplantation, and clinical parameters related to the administration of etanercept were reviewed retrospectively.
The allo-SCT was performed between June 2007 and March 2009 at Ajou University Hospital. The patients' characteristics are described in Table 1. As preparative regimens, two patients with severe aplastic anemia (SAA) were given cyclophosphamide (Cy) and thymoglobulin and five patients with acute leukemia or myelodysplastic syndrome (MDS) were given busulfan-cyclophosphamide (BuCy). Reduced intensity stem cell transplantation (RIST; busulfan + fludarabine + thymoglobulin) was used in eight patients, including one patient with comorbid disease and seven patients as salvage therapy after failure of a first transplantation (one patient with prior allo-SCT and the others with prior auto-SCT). Thymoglobulin was administered for RIST and for conditioning aplastic anemia.
The GVHD prophylaxis consisted of cyclosporin A (CsA; 3 mg/kg/day intravenously; Sandimun, Norvatis, Seoul, Korea) and a short course of methotrexate (MTX; 15 mg/m2 on D+1 and 10 mg/m2 on D+3, D+6, and D+11) in all cases; the MTX was omitted on D+11 in the RIST setting. If no GVHD developed, the CsA was to be tapered off by day 100. The first-line therapy for acute GVHD was a combination of CsA + steroid or FK506 + steroid at a dose equivalent to 2 mg/kg/day of methylprednisolone in nine and seven patients, respectively. The physicians changed the oral CsA to intravenous FK506 injection in the seven patients who could not take CsA easily on schedule. One myeloma patient who tapered off CsA rapidly after transplantation because of sustained M-protein was treated with steroid alone for his acute GVHD. The assessment and grading of acute GVHD was based on the clinical findings and followed previous reports [9,10,11].
Steroid refractoriness was defined as a progression of acute GVHD or no improvement in acute GVHD after at least 7 days of immunosuppressive therapy, including 2 mg/kg/day of methylprednisolone. Steroid-dependent patients were excluded from the analysis. The patients were given 25 mg of etanercept subcutaneously twice weekly for 4 weeks. The response was evaluated 4 weeks after starting the etanercept and the overall response was calculated as the sum of the organ-specific acute GVHD.
A CR was defined as the complete resolution of all manifestations of acute GVHD in all evaluable organs within 4 weeks of initiating the etanercept. A partial response (PR) was defined as a decrease in the severity of GVHD by at least 1 overall grade without deterioration of any organ system. New involvement of acute GVHD in any organ was considered progressive disease [8].
All patients were given prophylaxis with oral quinolone, itraconazole, and acyclovir starting with conditioning therapy until D+28. The daily administration of antituberculosis (rifampin and isoniazid) and an antiprotozoal drug (trimethoprim-sulfamethoxazole) was stopped immediately after the stem cell infusion and was changed to twice weekly after engraftment. Next, granulocyte-colony stimulating factor (G-CSF; Grasin, Kyowa Hakko Kirin, Tokyo, Japan) was given at a dose of 10 mg/kg/day subcutaneously to help the patients recover from neutropenia (absolute neutrophil count < 500/mm3). No antibiotics or antifungals were administrated for prophylaxis during or after etanercept therapy.
The GVHD grades before and after etanercept treatment were compared using the nonparametric Wilcoxon signed-rank test. The relationships between the clinical parameters and the response to etanercept were analyzed using logistic regression. All statistical analyses were conducted using SPSS version 13.0 (SPSS Inc., Chicago, IL, USA).
The median patient age was 43.5 years (range, 20 to 63), with males comprising 72.2% of the population. The transplantation data are shown in Table 1. There were seven patients with AML, four with multiple myeloma, three with SAA, two with acute lymphoblastic leukemia (ALL), and one patient each with MDS and chronic idiopathic myelofibrosis. The median interval from diagnosis to allo-SCT was 238.5 days. Fourteen patients received peripheral blood stem cells as a source of hematopoietic stem cells and unrelated transplantation was performed in nine patients. Transplantation from a female donor to a male patient occurred in eight cases. The initial treatment of acute GVHD was prednisone + FK506 in nine patients, prednisone + CsA in seven patients, and prednisone alone for one patient. The median days to starting etanercept from the documentation of steroid-refractoriness was 24 days and the median dose of etanercept was 100 mg (range, 25 to 200).
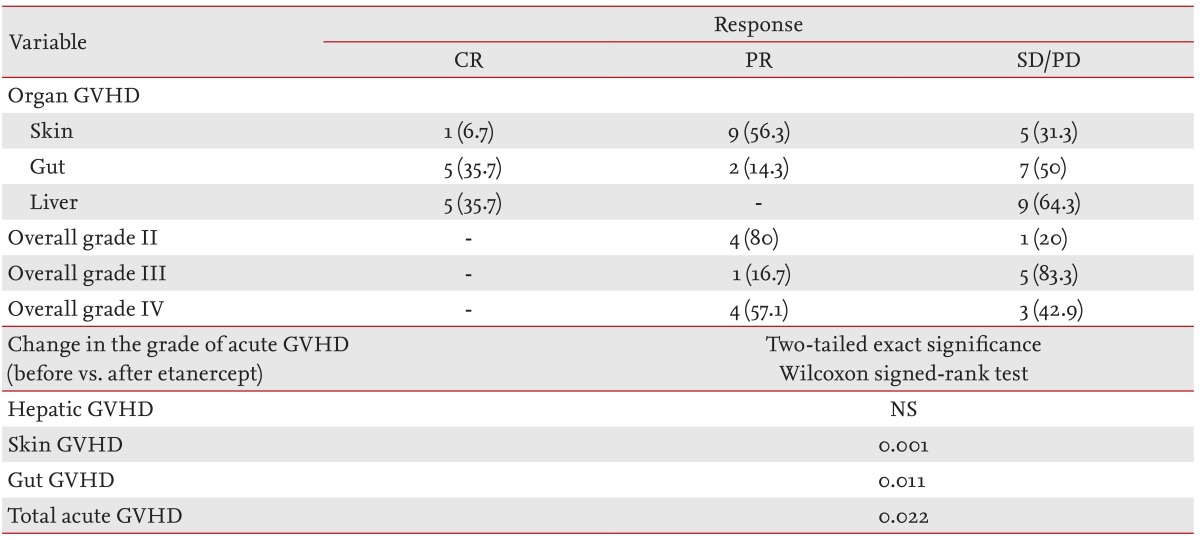
Eighteen patients who received etanercept to treat grade II to IV acute GVHD were enrolled in the analysis and were evaluable for response. Nine of the patients responded to etanercept (50% response rate). Thirteen patients had grade III or IV acute GVHD with a 38.5% response rate (5/13). Ten patients received four to eight of the planned eight doses (median four doses) of etanercept. Using a nonparametric test to compare before and after treatment, etanercept had a downgrading effect on acute GVHD (p = 0.022). The incidence of higher grade skin and gut GVHD decreased significantly after administering etanercept (p = 0.001 and p = 0.011, respectively), while the incidence of liver GVHD did not change. In terms of the overall grade of acute GVHD, no patient had a CR, while a PR occurred in 80% of the grade II patients and 57% of the grade IV patients. Only 17% of the patients with grade III acute GVHD responded to etanercept therapy (Table 2). One patient who received tandem allo-SCT died of acute GVHD and two of six patients with auto-allo-SCT are alive with improved acute GVHD grades and resulting limited chronic GVHD. The steroid dose could be reduced by more than 50% in eight of the nine patients who showed a clinical response.
Fifteen infections occurred in 12 patients. The lungs were the most common site of infection (8 of 15) and one case each of esophagitis, meningitis, and intra-abdominal abscess was documented. Candidemia was observed in two patients. Two patients developed cytomegalovirus reactivation that required pre-emptive ganciclovir treatment. Six patients experienced bleeding episodes, which were controlled without surgical intervention, except for one patient who died of an intracranial hemorrhage. The gastrointestinal tract was the most frequent site of bleeding.
Five of 18 patients are currently alive, with a median follow-up of 737.5 days (range, 497 to 1,139) after transplantation. Patients not responding to etanercept treatment had 100% mortality with a median survival of 92 days (range, 45 to 462) after transplantation, whereas the responders had a 55.6% survival rate. Five patients died of worsening acute GVHD, three patients died of a relapse of their underlying diseases (AML, n = 2; ALL, n = 1), and four patients died of pulmonary infection, including one patient who also had pulmonary aspergillosis. One patient died of an intracranial hemorrhage. In summary, five patients died of GVHD and three of them showed no response, resulting in the worsening of acute GVHD. Another two of five patients responded to etanercept initially, but eventually experienced a flared acute GVHD and subsequently died of extensive chronic GVHD.
No clinical parameter affected the response rate of etanercept, including patient characteristics, transplantation kinetics, acute GVHD outcomes, and various data related to etanercept. Disease status at transplantation did not affect the response rate to etanercept in the seven patients with AML. The statistical significance of disease status as a clinical parameter could not be analyzed in the patients with ALL, SAA, MDS, and myelofibrosis because of the extremely small sample sizes of the subgroups.
Our data demonstrated the clinical efficacy and safety of etanercept for treating steroid-refractory acute GVHD. The overall response rate was 50%, similar to that reported previously [2], but somewhat inferior to the response rate when combined with daclizumab [12]. There were several reasons for our low response rate. Only 10 of the 18 patients received a total etanercept dose of at least 100 mg. Five of the eight patients who received less than 100 mg did not have a chance to switch to other immunosuppressive drugs because of active infections. Another possible reason for the relatively low response rate might be because some of the patients were very high risk. Eight patients who underwent RIST had advanced disease having already experienced a failed conventional allo-SCT or auto-SCT. In addition, the median time to starting etanercept from the documentation of steroid refractoriness of acute GVHD was 17 days. The prompt administration of etanercept is believed to improve the response rate because the effect of etanercept at blocking the TNF-α receptor is worse when donor T cells are already activated [2,13].
Comparing the grade of acute GVHD before and after etanercept, skin and gut GVHD improved significantly (p < 0.001 and p = 0.011, respectively), while hepatic GVHD was not affected. As our data and reports show, the effectiveness of etanercept, a TNF-α blocker, can be limited to gut and skin GVHD [2,8,14]. This result might be due to the fact that hepatic GVHD is predominantly mediated by Fas ligand, while skin and gut GVHD are mediated by TNF-α [15].
The PR rate of grade IV patients (57.1%) was higher than that of grade III (17%). This was likely because six of the seven patients with grade IV acute GVHD had grade IV skin GVHD, whereas two of the six grade III patients had grade II-III hepatic GVHD.
We observed a steroid-sparing effect of etanercept; eight of nine patients who showed a clinical response achieved a steroid dose reduction exceeding 50%
All of the etanercept nonresponders died, while five of nine responders are still alive. In addition, two of the deaths in the responders group were due to the relapse of an underlying disease, sepsis and bleeding, and not to acute GVHD. In our series, the majority of patients died merely from worsening of the acute GVHD, without infectious complications. Three patients developed fungal infections (two cases of candidiasis and one case of invasive aspergillosis), resulting in one death. Infection was the second leading cause of death following acute GVHD itself, and fungal sepsis comprised 25% of all fatal infections. Since previous studies combined other immunosuppressive agents with etanercept to treat steroid-refractory acute GVHD, a direct comparison of infectious complications between those reports and our data is inappropriate [12,16]. However, compared with infectious mortalities of 52.4% (11/21) [12] and 31.3% (5/16) [17], our series had relatively low infectious mortality (22.2%, 4/18). We hope that prophylactic antifungal agents will reduce the mortality related to fungal infections.
A relatively low response rate combined with the relatively low mortality from infectious complications resulted in a 27.8% overall survival, which is comparable to the reports mentioned above. To treat steroid-refractory acute GVHD, especially grade III or IV, profound immunosuppression causes fatal complications that conceivably negate the advantage of controlling the acute GVHD. Of the seven patients with grade IV acute GVHD, four responded to etanercept and two are currently alive.
As stated to earlier, etanercept had a response rate similar to other second-line drugs, particularly in gut and skin GVHD, and relatively fewer complications related to infections. Etanercept is more expensive than nonspecific immunosuppressants such as MMF or tacrolimus; however, it prevents unwanted changes in many complicated in vivo pathways because it acts only at the TNF-α receptor. Thus etanercept can be administered to patients who are at risk of fatal infections.
In conclusion, although our study had several limitations, such as being a retrospective cohort review with a very small sample size, etanercept as a treatment for steroid-refractory acute GVHD appears to have a modest response rate, few fatal complications, and significant survival benefit. Further study of this immunosuppressive regimen in a larger patient cohort is needed.
References
1. MacMillan ML, Weisdorf DJ, Wagner JE, et al. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biol Blood Marrow Transplant 2002;8:387–394PMID : 12171485.


2. Levine JE, Paczesny S, Mineishi S, et al. Etanercept plus methylprednisolone as initial therapy for acute graft-versus-host disease. Blood 2008;111:2470–2475PMID : 18042798.



3. Kim DH, Sohn SK, Baek JH, et al. Retrospective multicenter study of allogeneic peripheral blood stem cell transplantation followed by reduced-intensity conditioning or conventional myeloablative regimen. Acta Haematol 2005;113:220–227PMID : 15983427.


4. Weisdorf D, Haake R, Blazar B, et al. Treatment of moderate/severe acute graft-versus-host disease after allogeneic bone marrow transplantation: an analysis of clinical risk features and outcome. Blood 1990;75:1024–1030PMID : 2302454.


5. Basara N, Blau WI, Romer E, et al. Mycophenolate mofetil for the treatment of acute and chronic GVHD in bone marrow transplant patients. Bone Marrow Transplant 1998;22:61–65PMID : 9678797.


6. Arai S, Margolis J, Zahurak M, Anders V, Vogelsang GB. Poor outcome in steroid-refractory graft-versus-host disease with antithymocyte globulin treatment. Biol Blood Marrow Transplant 2002;8:155–160PMID : 11939605.


7. Markey KA, Burman AC, Banovic T, et al. Soluble lymphotoxin is an important effector molecule in GVHD and GVL. Blood 2010;115:122–132PMID : 19789388.


8. Uberti JP, Ayash L, Ratanatharathorn V, et al. Pilot trial on the use of etanercept and methylprednisolone as primary treatment for acute graft-versus-host disease. Biol Blood Marrow Transplant 2005;11:680–687PMID : 16125638.


9. Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. diagnosis and staging working group report. Biol Blood Marrow Transplant 2005;11:945–956PMID : 16338616.


10. Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on acute GVHD grading. Bone Marrow Transplant 1995;15:825–828PMID : 7581076.

11. Bacigalupo A. Management of acute graft-versus-host disease. Br J Haematol 2007;137:87–98PMID : 17391488.


12. Wolff D, Roessler V, Steiner B, et al. Treatment of steroid-resistant acute graft-versus-host disease with daclizumab and etanercept. Bone Marrow Transplant 2005;35:1003–1010PMID : 15806135.


13. Maeda Y, Levy RB, Reddy P, et al. Both perforin and Fas ligand are required for the regulation of alloreactive CD8+ T cells during acute graft-versus-host disease. Blood 2005;105:2023–2027PMID : 15466930.


14. Herve P, Flesch M, Tiberghien P, et al. Phase I-II trial of a monoclonal anti-tumor necrosis factor alpha antibody for the treatment of refractory severe acute graft-versus-host disease. Blood 1992;79:3362–3368PMID : 1596576.


15. Hattori K, Hirano T, Miyajima H, et al. Differential effects of anti-Fas ligand and anti-tumor necrosis factor alpha antibodies on acute graft-versus-host disease pathologies. Blood 1998;91:4051–4055PMID : 9596649.


Table 1
Patients' characteristics
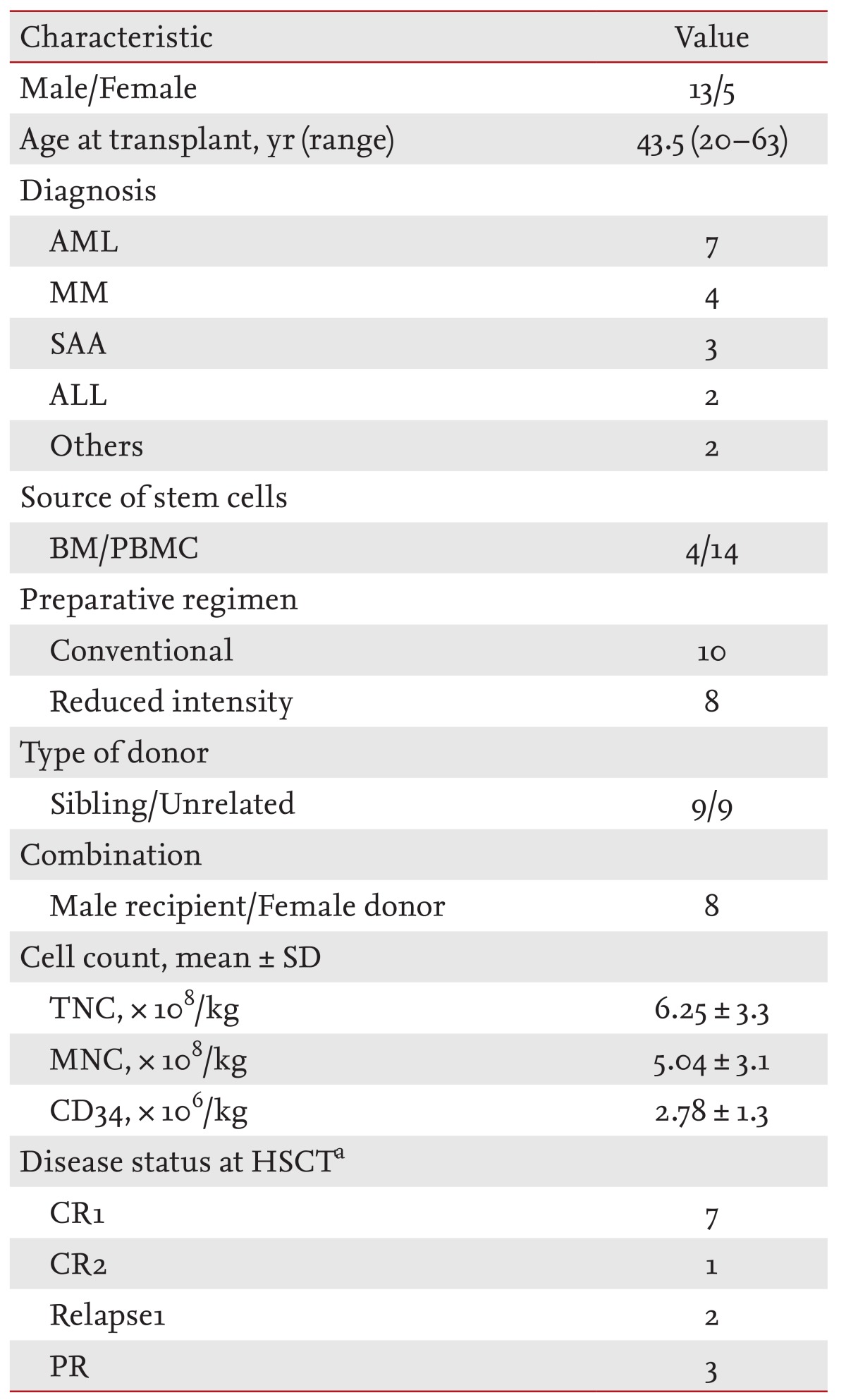
The majority of the population had a normal karyotype using the G-banding technique. All of the patients received stem cells from HLA-identical donors and there was no failed engraftment. Almost 100% donor chimerism was achieved.
AML, acute myeloid leukemia; MM, multiple myeloma; SAA, severe aplastic anemia; ALL, acute lymphoblastic leukemia; BM, bone marrow; PBMC, peripheral blood mononuclear cell; TNC, total nucleated cell; MNC, mononuclear cell; HSCT, hematopoietic stem cell transplantation; CR, complete response; PR, partial response.
aCases of acute leukemia and multiple myeloma were included.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



