INTRODUCTION
Enterobacter species are important nosocomial pathogens that have been increasingly reported to be associated with intra-abdominal, respiratory tract, urinary tract, wound, and bloodstream infections in clinical practice [
1,
2]. These bacteria are characterized by chromosomally-encoded AmpC ╬▓-lactamases and can develop antimicrobial resistance on exposure to broad-spectrum cephalosporins [
3,
4]. Moreover, recent studies have suggested the global emergence of extended-spectrum ╬▓-lactamase (ESBL)-producing
Enterobacter spp. [
5,
6,
7,
8,
9,
10,
11,
12]. This emergence poses a serious public health threat, since only a limited number of effective antimicrobial agents can be used against multidrug-resistant organisms producing both an ESBL and AmpC ╬▓-lactamase.
In patients with malignancy, who require effective antimicrobial therapy early in the course of their infection, inappropriate antimicrobial therapy due to ESBL-producing Enterobacter spp. may have a serious adverse impact on their clinical outcome. However, to the best of our knowledge, no reports have yet described the prevalence of ESBL-producing Enterobacter spp. and the impact of ESBL production on the clinical outcome of patients with malignancy.
This study investigated the prevalence and clinical impact of ESBL production in cancer patients with Enterobacter spp. bacteremia.
METHODS
This study follows several prospective studies performed by our group regarding AmpC ╬▓-lactamase-producing
Enterobacteriaceae, including
Enterobacter spp.,
Serratia marcescens, Citrobacter freundii, and
Morganella morgannii [
4,
5,
13]. We prospectively identified all patients admitted to Asan Medical Center, a 2,200-bed tertiary care university-affiliated teaching hospital in Seoul, Korea, with clinical specimens yielding AmpC ╬▓-lactamase-producing
Enterobacteriaceae. Patient clinical data were collected, including demographic characteristics, underlying diseases or conditions, initial manifestations, antimicrobial therapies, and outcomes. The present study focused on episodes of
Enterobacter spp. bacteremia diagnosed in patients with malignancy between January 2005 and November 2008. Recurrent episodes of
Enterobacter bacteremia in the same patient were included only if the time interval between two episodes was more than 28 days, or if different
Enterobacter species were responsible for each episode.
Enterobacter spp. were identified using an NEG Combo Panel Type 32 (Dade Behring, Deerfield, IL, USA), and antimicrobial susceptibility was examined using the MicroScan system (Dade Behring) and standard criteria of the Clinical and Laboratory Standards Institute [
14]. ESBL production was assessed by the double-disk synergy test. Briefly, the inhibitory zone diameters of disks containing 30 ┬Ąg of cefatoxime, ceftazidime, or cefepime, either alone, or in combination with 10 ┬Ąg of clavulanate, were compared. An ESBL production phenotype was defined as a Ōēź 5 mm increase in zone diameter for at least one of the combination disks relative to its corresponding single antibiotic disk [
4,
15].
Escherichia coli strain ATCC 25922 and
Klebsiella pneumoniae strain ATCC 700603 were used as negative and positive controls for ESBL production, respectively. Several ESBL-encoding genes (
blaTEM,
blaSHV, bla
CTX-M,
blaVEB,
blaGES, and
blaPER) were detected by polymerase chain reaction (PCR) using primers based on published sequences, followed by DNA sequencing [
8,
11,
12,
16].
A patient was considered to have malignancy if he or she had been treated for malignancy within three years prior to developing bacteremia. The McCabe-Jackson criteria, Charlson's comorbidity index scores, and systemic inflammatory response syndrome criteria were defined as described previously [
17,
18,
19]. A hospital-acquired infection was defined as bacteremia occurring more than 48 hours after admission. The source of infection was defined only if it had been clinically or microbiologically documented. Prior usage of antimicrobial agents was defined as the receipt of a systemic antimicrobial agent for at least 48 hours within one month prior to the onset of bacteremia. Third-generation cephalosporins, including ceftriaxone, cefotaxime, and ceftazidime, were defined as extended-spectrum cephalosporins (ESCs). Appropriate antimicrobial therapy was defined as the receipt of
in vitro active antimicrobial agents for at least 48 hours against the identified isolates.
A statistical analysis was performed using SPSS version 18.0 (IBM Co., Armonk, NY, USA). Binary data were compared using the chi-square or Fisher exact test, and continuous scaled data were compared using the Student t test or Mann-Whitney U test. A logistic regression analysis was performed to investigate independent risk factors for 30-day mortality. Variables with a p value < 0.1 in univariate analyses were included in the logistic regression analysis. A p value < 0.05 was considered significant.
RESULTS
During the study period, 255 episodes of
Enterobacter spp. bacteremia occurred in patients with malignancy. After excluding 52 episodes for which
Enterobacter isolates were not available, 203 episodes of
Enterobacter spp. bacteremia were finally included in the analysis. More than half of the study patients were male (113/203, 55.7%). The median patient age was 57 years. Except for malignancy, biliary tract disease was the most common underlying disease (36/203, 17.7%), followed by diabetes mellitus (26/203, 12.8%) and liver cirrhosis (17/203, 8.4%). The majority of infections (166/203, 81.8%) were hospital-acquired.
Enterobacter cloacae isolates were responsible for the majority (145/203, 71.4%) of infections, and more than a quarter were caused by
Enterobacter aerogenes (53/203, 26.1%). Nearly half (48.8%, 99/203) were caused by ESC-resistant isolates. ESBL production was detected in 31 isolates (15.3%). Inappropriate empirical antimicrobial therapy was observed in 24 patients (11.8%). A total of 34 patients died within 30 days after
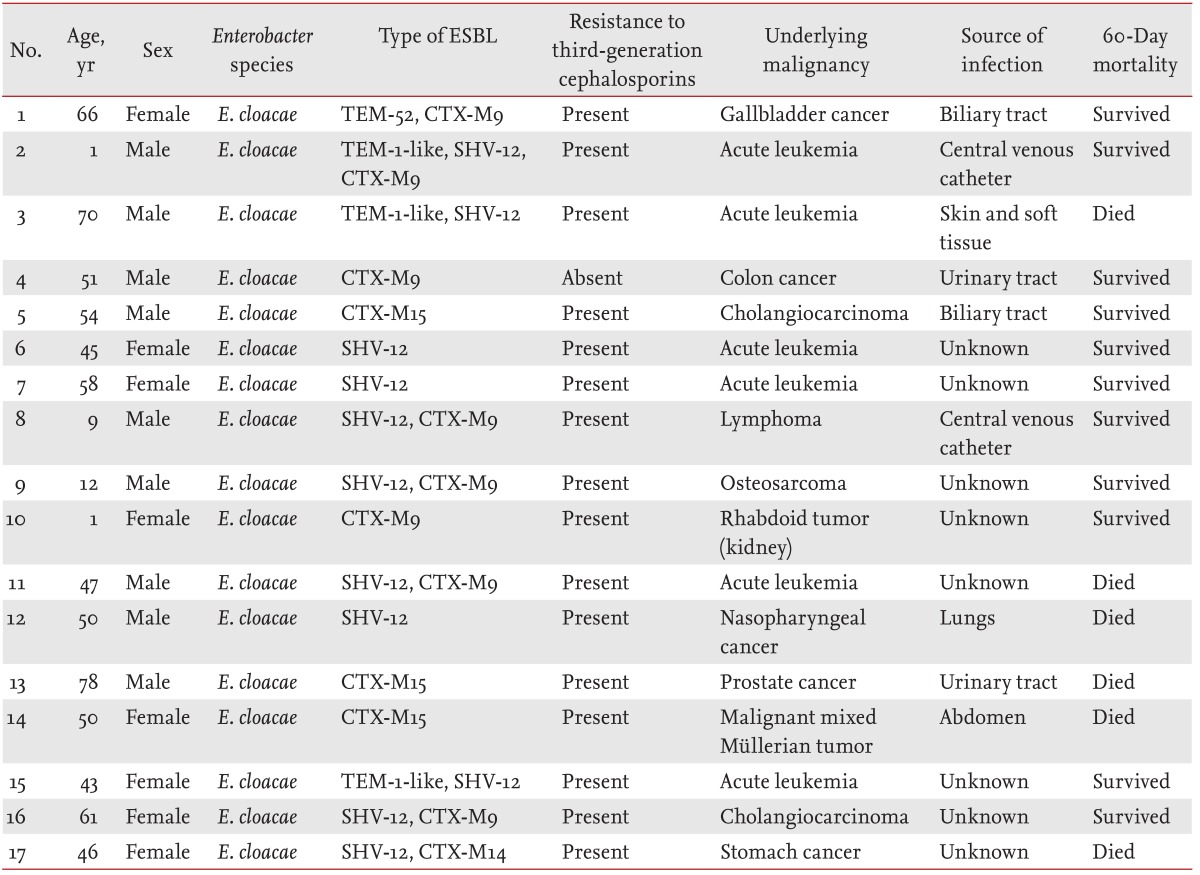
Enterobacter spp. bacteremia (16.7%), and the median length of hospital stay was 28 days. Of the 31 ESBL-producing isolates, 28 were used for the detection of ESBL genes. ESBL genes were detected in 17 cases: 12 were positive for CTX-M (eight CTX-M9, three CTX-M15, and one CTX-M14), 11 for SHV-12, and four for TEM (three TEM-1-like and one TEM-52) (
Table 1). PER, VEB, and GES genes were not detected in any of the cases.
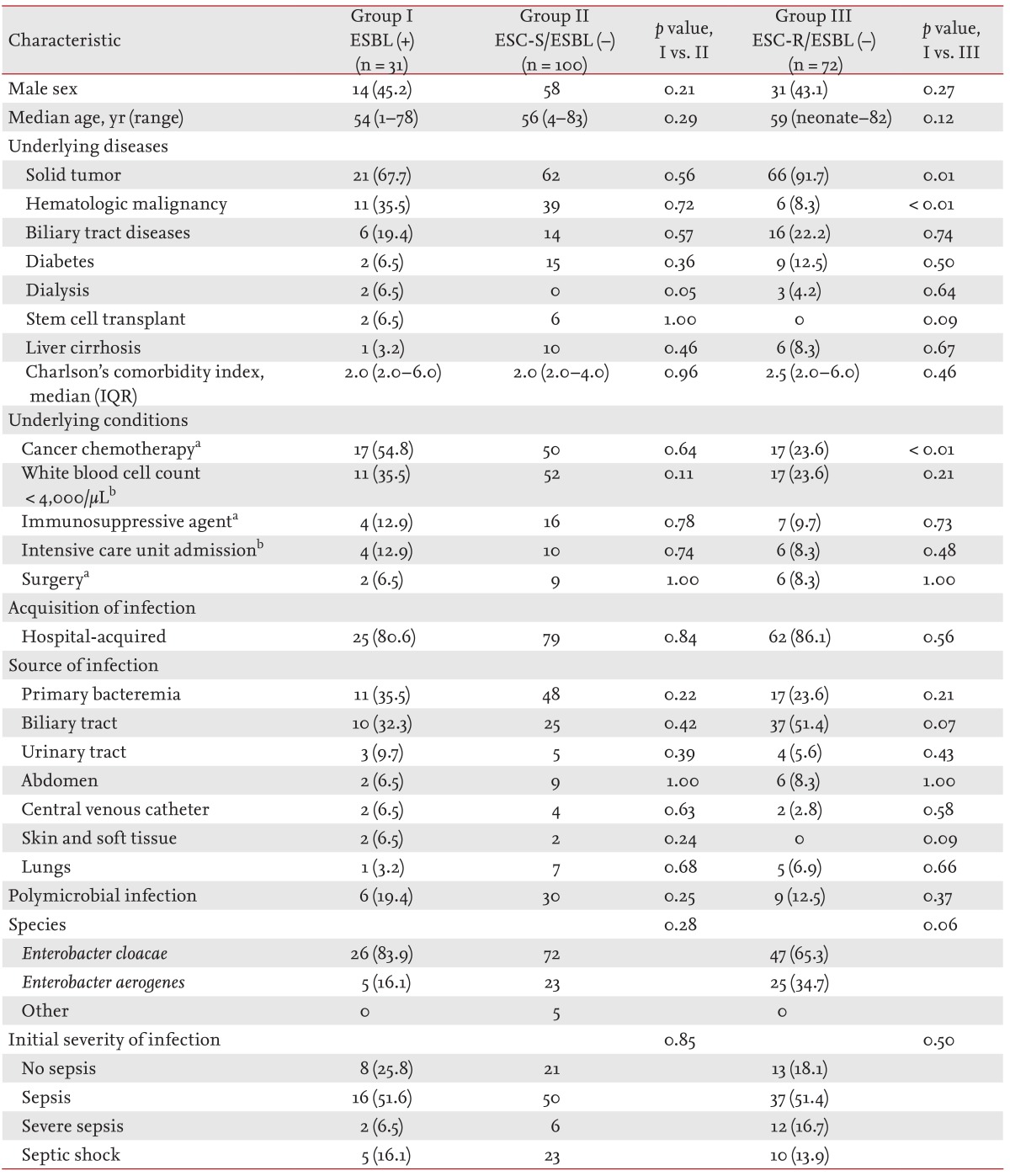
The clinical characteristics of the patients with
Enterobacter bacteremia episodes caused by ESBL-producing isolates (ESBL-positive group) were compared to those of the patients with ESC-susceptible non-ESBL-producing blood isolates (ESC-S/ESBL-negative group). The ESBL-positive and ESC-S/ESBL-negative groups did not differ in their demographic characteristics or underlying diseases/conditions. The acquisitions, sources, and initial severities of infection were also not different between the two groups (
Table 2). Prior usage of antimicrobial agents was more common in the ESBL-positive group (77.4% vs. 54.0%,
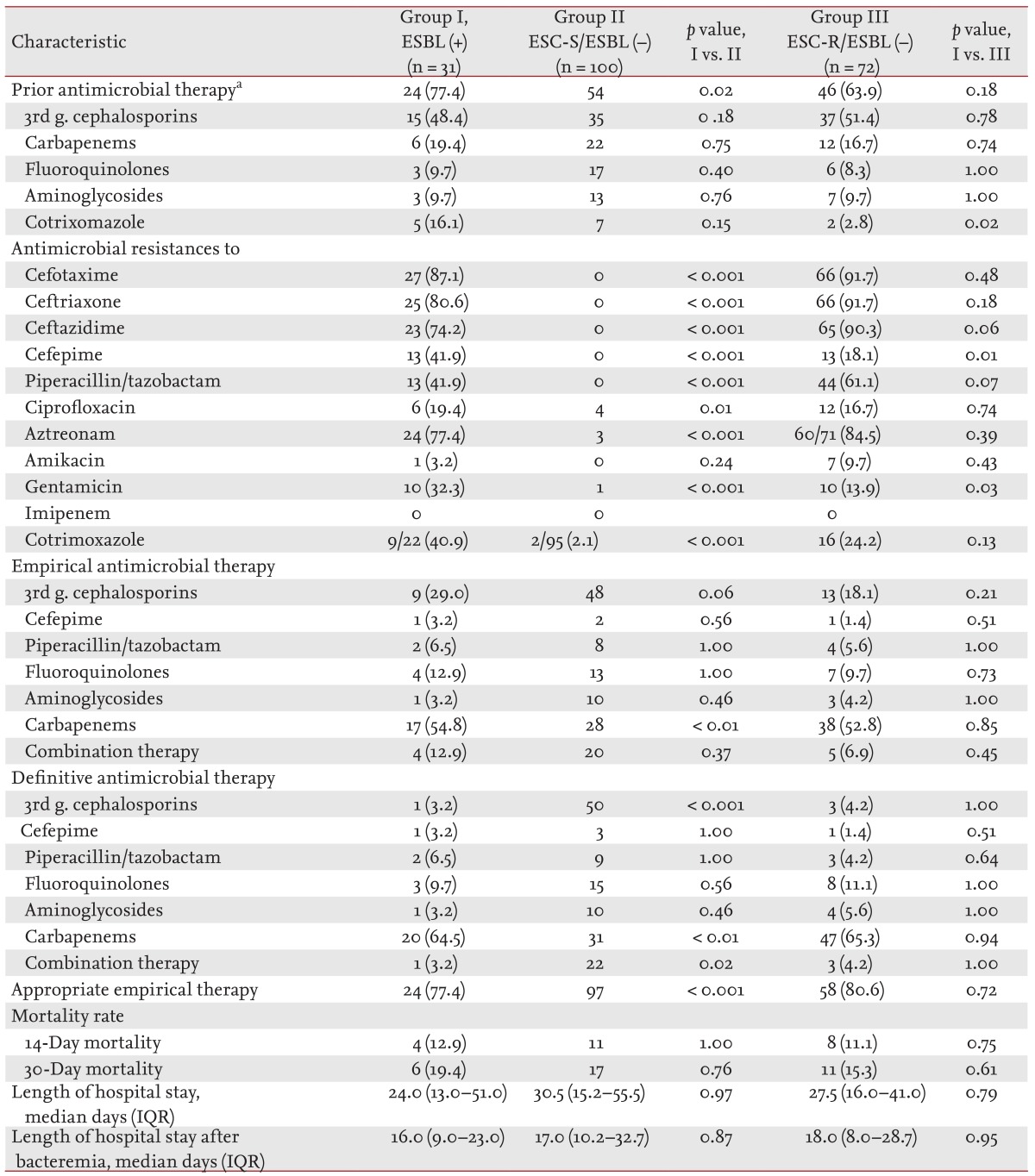
p = 0.02). The rates of antimicrobial resistance were higher in the ESBL-positive group than in the ESC-S/ESBL-negative group, except for the rate of resistance to amikacin. Carbapenems were more frequently used in the ESBL-positive group as empirical (54.8% vs. 28.0%,
p < 0.01) or definitive therapy (64.5% vs. 31.0%,
p < 0.01), whereas ESCs were more frequently used in the ESC-S/ESBL-negative group as empirical (48.0% vs. 29.0%,
p < 0.06) or definitive therapy (50.0% vs. 3.2%,
p < 0.001). The ESBL-positive group received more inappropriate empirical antimicrobial therapies than the ESC-S/ESBL-negative group (22.6% vs. 3.0%,
p < 0.001). The mortalities and median lengths of hospital stay of the two groups did not differ (
Table 3).
The clinical characteristics of the ESBL-positive group were also compared to those of patients with ESC-resistant non-ESBL-producing blood isolates (ESC-R/ESBL-negative group), which were thought to produce AmpC ╬▓-lactamases. The two groups did not differ in their demographic characteristics. Hematologic malignancy (35.5% vs. 8.3%,
p < 0.01) and recent chemotherapy (54.8% vs. 23.6%,
p < 0.01) were more common in the ESBL-positive group. The acquisition, sources, and initial severities were not different between the two groups (
Table 2). Prior usage of trimethoprim-sulfamethoxazole (TMP-SMX) was more common in the ESBL-positive group (16.1% vs. 7.0%,
p = 0.02). The rate of antimicrobial resistance to cefepime (41.9% vs. 18.1%,
p = 0.01) and gentamicin (32.3% vs. 13.9%,
p = 0.03) was higher in the ESBL-positive group than in the ESC-R/ESBL-negative group, respectively. The two groups did not differ in antimicrobial therapy, mortality, and median length of hospital stay (
Table 3).
To investigate possible risk factors for 30-day mortality, univariate analyses were performed (
Table 4). Independent risk factors for 30-day mortality included initial presentation of severe sepsis or septic shock (adjusted odds ratio [AOR], 2.72; 95% confidence interval [CI], 1.16 to 6.36;
p = 0.02), pneumonia (AOR, 6.46; 95% CI, 1.69 to 24.76;
p = 0.01), and intra-abdominal infection (AOR, 8.67; 95% CI, 2.61 to 28.80;
p < 0.001).
DISCUSSION
Based on the results of the phenotypical ESBL tests, ESBL-producing isolates were responsible for 15.3% of the Enterobacter bacteremia episodes that occurred in patients with malignancy in this study. Prior usage of antimicrobial agents was more common in the ESBL-positive group than in the ESC-S/ESBL-negative group. Although the ESBL-positive group received inappropriate empirical antimicrobial therapy more often than the ESC-S/ESBL-negative group, the clinical outcomes of the two groups did not differ. Hematologic malignancy, recent chemotherapy, and prior use of TMP-SMX were more common in the ESBL-positive group than in the ESC-R/ESBL-negative group.
Previous phenotypical ESBL tests have shown that the ESBL production rate among
Enterobacter spp. from clinical specimens ranged from 12.8% to 43.0% [
5,
6,
7,
8,
9,
10,
11,
12]. Several clinical studies regarding ESBL production in blood isolates from
Enterobacter infections have also been published [
6,
7,
12,
20,
21,
22]. In a Korean study, ESBL production was observed in 43% of all blood isolates of
Enterobacter spp. and 67.3% of all ESC-resistant isolates, suggesting that ESBL production is common among
Enterobacter spp. [
7]. More recently, in Korea and Taiwan, phenotypical ESBL production was found in 24 out of 99 blood isolates of
Enterobacter spp. (23.4%) and in 123 out of 610 blood isolates of
E. cloacae (20.2%), respectively [
20,
21]. In a recent study in the United States, 16 out of 159 blood isolates of
E. cloacae produced ESBLs, as assessed by genotypic ESBL tests (10.1%) [
22]. In this study, 15.3% of all blood isolates of
Enterobacter spp. were ESBL-producers, as determined by the phenotypic ESBL test. To the best of our knowledge, this is the first study to evaluate ESBL production among blood isolates of
Enterobacter spp. obtained from patients with malignancy. Considering the relatively low rate of ESBL production (15.3%) observed in this study, carbapenems may be infrequently required for
Enterobacter spp. bacteremia in patients with malignancy, contrary to expectations inferred from the higher positive rates in other recent studies. However, the ESBL production rate may vary according to the regional epidemiology of resistance, the characteristics of the patients admitted to a hospital, and the effectiveness of infection control measures at each care center. Thus, the ESBL production rate among blood isolates of
Enterobacter spp. should be investigated in clinical studies from variable geographic regions and in different clinical settings; moreover, the ESBL production rate should be monitored continuously, especially in patients with malignancy.
In this study, previous antimicrobial therapy was more common in the ESBL-positive group than in the ESC-S/ESBL-negative group. Previous antimicrobial therapy is regarded as one of the most important risk factors for antibiotic-resistant gram-negative bacilli infections in patients with cancer [
23,
24]. Previous antimicrobial therapy has also been noted as a risk factor for ESBL production in studies of blood isolates of
Enterobacter spp. [
20,
21,
22].
Despite more frequent inappropriate empirical antimicrobial therapy, the mortality rate and the median length of hospital stay in the ESBL-positive group did not differ from those in the ESC-S/ESBL-negative group. In a recent study of AmpC ╬▓-lactamase-producing
Enterobacteriaceae, in which 75.8% of all study isolates were
Enterobacter spp., inappropriate initial antimicrobial therapy occurred more frequently in the ESBL group, but the 30-day mortality rate did not differ between the ESBL and non-ESBL groups. However, the average length of the hospital stay after bacteremia was longer in the ESBL group [
20]. That study, by Cheong et al. [
20], differed from ours in that malignancy was observed in only 58.9% of the study patients and inappropriate initial antimicrobial therapy was common in the ESBL group (63.3%) compared with our study (22.6%). Patients with malignancy tended to receive broad-spectrum antimicrobial agents as empirical antimicrobial therapies more frequently than those without malignancy. This tendency could have masked the adverse impact of ESBL production in our study. Another recent study also investigated the impact of ESC resistance on the clinical outcomes of adult cancer patients with
Enterobacter spp. bacteremia, although the underlying mechanism of the resistance (e.g., ESBL or AmpC ╬▓-lactamase production) was not provided. The ESC-resistant group had a higher 30-day mortality rate than the ESC-susceptible group (30.8% vs. 16.4%,
p = 0.03) [
25]. These results are not consistent with our findings, in which the 30-day mortality rate was not different between ESC-susceptible and -resistant patients (17.3% vs. 16.2%,
p = 0.67). Although the characteristics of the study patients were similar to ours, antimicrobial resistance to ESCs (27.6% vs. 48.8%) and the empirical use of broad-spectrum antimicrobial agents such as carbapenems (23.1% vs. 54.5%) were less common in that study [
25] than in ours, respectively. At our study center, since ESC-resistant isolates were encountered relatively commonly, the empirical use of broad-spectrum antimicrobial agents was frequent. At least in part, this might be responsible for the lack of difference in the 30-day mortality rate observed in our study. The clinical impact of ESC resistance associated with ESBL production in cancer patients with
Enterobacter bacteremia requires further investigation. On the other hand, our explanation for the lack of differences seen in the clinical outcomes should not be taken to imply that broad-spectrum antimicrobial agents such as carbapenems are necessary as an empirical antimicrobial therapy for patients with malignancy to reduce the adverse impact of ESBLs. As a result of indiscriminate antimicrobial therapy, many clinicians have already encountered extremely multidrug-resistant Gram-negative bacilli such as carbapenemase-producing
Enterobacteriaceae, carbapenem-resistant
Acinetobacter baumannii, and carbapenem-resistant
Pseudomonas aeruginosa in clinical practice [
26,
27]. We may need to introduce more sophisticated therapeutic strategies, including de-escalation therapy or combination therapy with non-╬▓-lactam antimicrobial agents, as empirical antimicrobial therapies.
The ESBL-positive group was also compared with the ESC-R/ESBL-negative group, which was thought to produce AmpC ╬▓-lactamase. Similarly, in a recent study of adult patients with
E. cloacae bacteremia, the clinical characteristics of ESBL and cefotaxime-nonsusceptible non-ESBL groups were compared. The ESBL group had more episodes of hospital-onset and polymicrobial bacteremia, an increased severity of illness, and longer stays in hospital, but the mortality rates were similar between the two groups [
21]. Their results are not directly comparable to ours because only 34.9% of the study patients had malignancy and there were methodological differences as well. Our study showed that hematologic malignancy, recent chemotherapy, and prior use of TMP-SMX were more common in the ESBL-positive group. Patients with hematologic malignancy are readily exposed to broad-spectrum antimicrobial agents due to frequent and prolonged neutropenia after bone marrow-toxic chemotherapy, for which the prophylactic use of TMP-SMX is commonly performed. Additionally, patients with cancer undergoing active chemotherapy may have more chance of being colonized or infected with antimicrobial-resistant bacteria compared with those who are not because they have more contact with healthcare workers and/or increased immune suppression. However, these risk factors for ESBL production over possible AmpC ╬▓-lactamase producers should be clarified in future studies.
There are several important limitations to our study. First, since our study was performed at a single large medical center our results may not be entirely applicable to different hospital settings. Second, the ESBL phenotypic test was not comprehensively performed for all consecutive Enterobacter spp. blood isolates, only for 79.6%. Third, the detection of ESBL genes by PCR was not performed for all ESBL-positive isolates. Fourth, pediatric and adult patient data were combined in this analysis, due to the limited number of patients with ESBL-positive Enterobacter bacteremia. However, only 6.9% of the patients were pediatric patients.
In conclusion, ESBL production was found in 15.3% of all Enterobacter spp. blood isolates obtained from patients with malignancy; moreover, inappropriate initial antimicrobial therapy was more commonly observed in the ESBL-positive group. However, ESBL production was not associated with poorer outcomes in patients with cancer and Enterobacter bacteremia.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





