Abstract
Background/Aims
To evaluate the impact on mortality of anti-tumor necrosis factor (anti-TNF) treatment of rheumatoid arthritis-associated interstitial lung disease (RA-ILD).
Methods
We retrospectively reviewed the medical records of 100 RA-ILD patients who visited our tertiary care medical center between 2004 and 2011, identified those treated with an anti-TNF agent, divided patients into non-survivor and survivor groups and evaluated their clinical characteristics and causes of death.
Results
A total of 24 RA-ILD patients received anti-TNF therapy, of whom six died (25%). Mean age at initiation of anti-TNF therapy was significantly higher in the nonsurvivor versus survivor group (76 years [range, 66 to 85] vs. 64 years [range, 50 to 81], respectively; p = 0.043). The mean duration of anti-TNF treatment in the non-survivor group was shorter (7 months [range, 2 to 14] vs. 23 months [range, 2 to 58], respectively; p = 0.030). The duration of anti-TNF therapy in all nonsurviving patients was < 12 months. Pulmonary function test results at ILD diagnosis, and cumulative doses of disease-modifying drugs and steroids, did not differ between groups. Five of the six deaths (83%) were related to lung disease, including two diffuse alveolar hemorrhages, two cases of acute exacerbation of ILD, and one of pneumonia. The sixth patient died of septic shock following septic arthritis of the knee.
Conclusions
Lung complications can occur within months of initial anti-TNF treatment in older RA-ILD patients; therefore, anti-TNF therapy should be used with caution in these patients.
Keywords: Arthritis, rheumatoid; Lung diseases, interstitial; Adalimumab; TNFR-Fc fusion protein; Infliximab
INTRODUCTION
Rheumatoid arthritis-associated interstitial lung disease (RA-ILD), the most common manifestation of rheumatoid lung disease [
1], occurs more frequently in patients with severe RA. An autopsy study of 81 patients with longstanding RA revealed that 16% died of respiratory failure, while 34% exhibited signs of ILD [
2]. Based on United States national mortality statistics, the prevalence of RA-ILD in RA patients is approximately 10% in females and 6% in males [
3].
Despite possible adverse events, including infection, the efficacy of anti-tumor necrosis factor (anti-TNF) therapy for the treatment of RA has been established in several randomized controlled trials [
4]. However, the effect of anti-TNF therapy on RA-ILD patients remains unclear. Improvements in pulmonary function, and radiographic stabilization, have been observed in RA patients following anti-TNF therapy [
5]; however, ILD exacerbation following administration of an anti-TNF agent has also been reported [
6,
7]. Moreover, the mortality odds ratio (OR) was increased 4.4-fold in RA patients with pre-existing lung disease treated with biological agents versus those without lung disease [
8]. The present, retrospective study investigates the causes of, and risk factors for, death in RA-ILD patients treated with anti-TNF agents.
METHODS
The medical records of 100 RA-ILD patients, treated in our tertiary care center between June 2004 and June 2011, were reviewed retrospectively. A total of 24 patients treated with anti-TNF therapy was selected. All patients were diagnosed according to the 1987 American College of Rheumatology (formerly the American Rheumatism Association) classification criteria for RA [
9]. The study was approved by the Institutional Review Board of Asan Medical Center.
Patients exposed to environmental agents or drugs, or with other underlying disorders known to cause pulmonary fibrosis, were excluded. RA-ILD was diagnosed by a pulmonologist based on a combination of clinical presentation, pulmonary function testing, and the presence of bibasilar reticular abnormalities with minimal ground-glass opacities, on high-resolution computed tomography. In certain cases, bronchoscopy with bronchoalveolar lavage was performed.
The baseline characteristics of patients were assessed. In addition, the nonsurvivor and survivor groups were compared to determine the effects of anti-TNF therapy on mortality. Data pertaining to age at diagnosis of RA-ILD, sex, disease duration, comorbidities, lung function, treatment regimen, and the number of acute exacerbations of ILD were also analyzed; patients who died were studied in detail.
Baseline patient characteristics were compared using either a Mann-Whitney U or chi-squared test. Group comparison of ORs and corresponding 95% confidence intervals (CIs), for each variable, were calculated using univariate and multivariate analyses. A value of p < 0.05 was taken to indicate statistical significance. All analyses were performed using the SPSS version 17.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
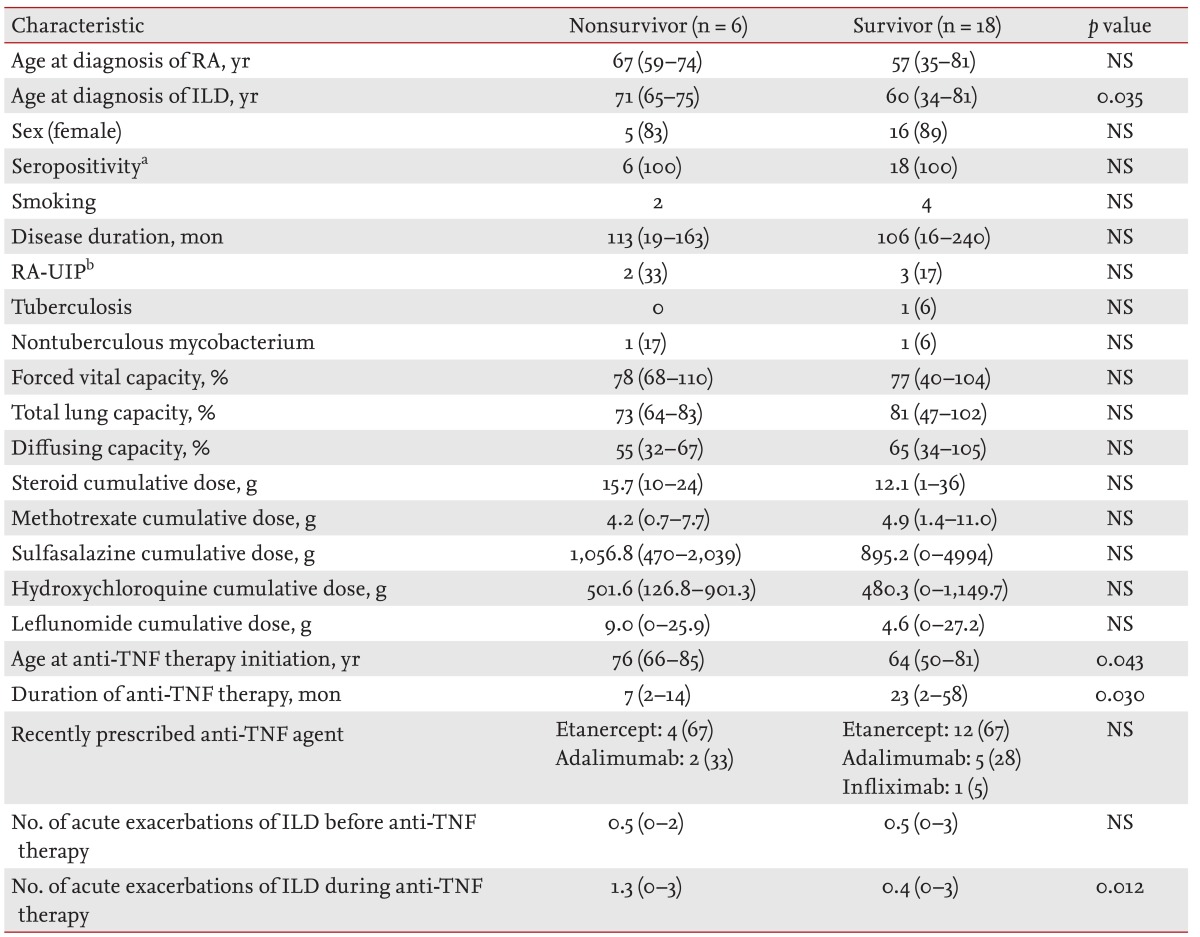
Of the 24 patients treated with anti-TNF therapy, six died (25%). The mean age at RA diagnosis was not significantly higher in the nonsurvivor versus survivor group (67 years [range, 59 to 74] vs. 57 years [range, 35 to 81], respectively) (
Table 1). However, mean age at ILD diagnosis was significantly higher in patients who died (71 years [range, 65 to 75] vs. 60 years [range, 34 to 81], respectively;
p = 0.035). There were more females in both groups and all patients were diagnosed as seropositive for RA. RA disease durations were similar between survivors and non-survivors (113 months [range, 19 to 163] vs. 106 months [range, 16 to 240], respectively). Five patients (three survivors and two nonsurvivors) were diagnosed with RA-UIP. Because no RA-ILD patients had undergone lung biopsy, their diagnoses were made according to clinical symptoms and imaging findings. Tuberculosis infection developed in one survivor, and one case of nontuberculous mycobacterium infection was observed in each group.
Pulmonary function test results at ILD diagnosis were analyzed, with no differences in the forced vital capacity of nonsurvivors versus survivors (78% [range, 68 to 110] vs. 77% [range, 40 to 104], respectively), nor total lung capacity (73% [range, 64 to 83] vs. 81% [range, 47 to 102], respectively), or diffusing capacity (55% [range, 32 to 67] vs. 65% [range, 34 to 105], respectively).
The cumulative dose of drugs, including steroids, methotrexate, sulfasalazine, hydroxychloroquine, and leflunomide was similar in both groups. However, mean age at anti-TNF therapy initiation was significantly higher in the nonsurvivor group (76 years [range, 66 to 85] vs. 64 years [range, 50 to 81], respectively; p = 0.043). The mean duration of anti-TNF treatment in the nonsurvivor group was shorter (7 months [range, 2 to 14] vs. 23 months [range, 2 to 58], respectively; p = 0.030). In both groups, the most common recently prescribed anti-TNF agent was etanercept.
Despite the shorter duration of anti-TNF treatment, a higher number of acute exacerbations of ILD following anti-TNF therapy was observed in the nonsurvivor versus survivor group (1.3 [range, 0 to 3] vs. 0.4 [range, 0 to 3], respectively; p = 0.012). However, there was no group difference in the number of acute exacerbations prior to anti-TNF therapy.
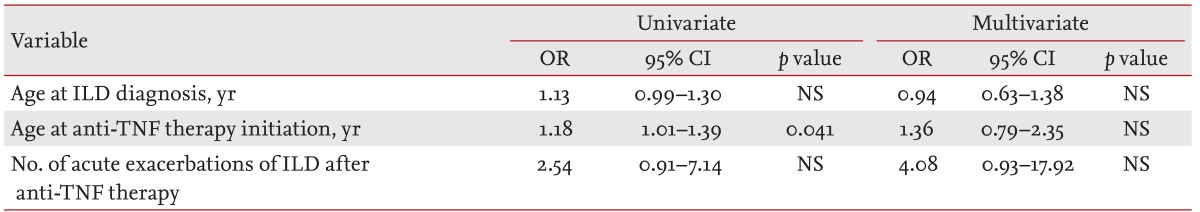
We analyzed risk factors for death using logistic regression (
Table 2). In univariate analysis, the only risk factor for death was age at initiation of anti-TNF therapy (OR, 1.18; 95% CI, 1.01 to 1.39;
p = 0.041). In multivariate analysis, no significant risk factor for death emerged.
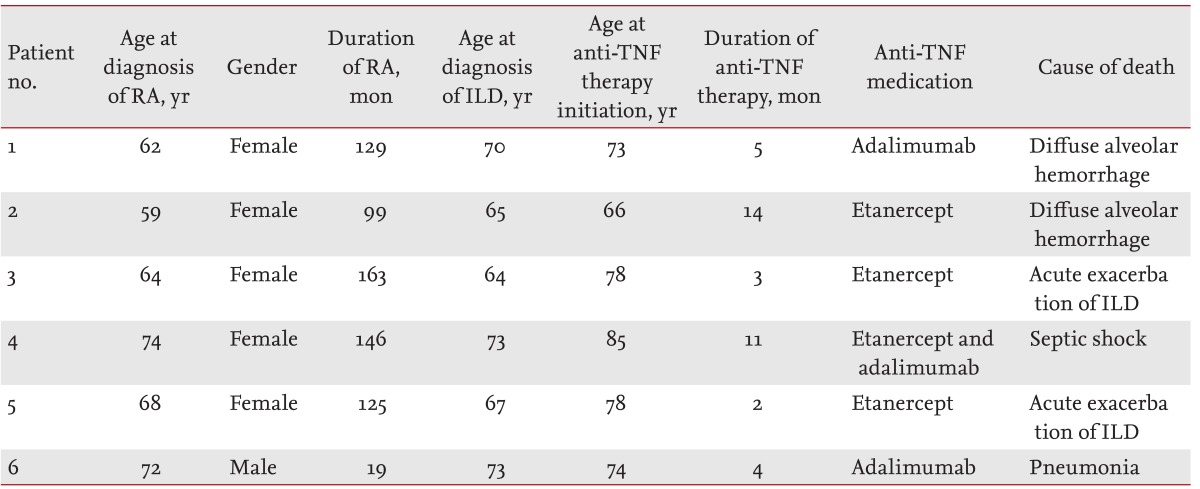
Patient clinical characteristics and causes of death are listed in
Table 3; age at ILD diagnosis and initiation of anti-TNF therapy was Ōēź 64 years. Anti-TNF treatment duration ranged between 2 to 14 months. Patients 1 and 2, who died of diffuse alveolar hemorrhage, were treated with etanercept and adalimumab, respectively. Patient 3, who received adalimumab for 2 months following 9 months of etanercept treatment, died of septic shock following knee surgery. Patient 4, treated with etanercept for 3 months, died of acute exacerbation of ILD following knee surgery. Patients 5 and 6, who died of acute exacerbation of ILD and pneumonia, were treated with etanercept and adalimumab, respectively.
DISCUSSION
The safety and effectiveness of anti-TNF agents, for the treatment of RA, have been well-established in clinical trials and large cohort studies. The number of RA-ILD patients treated with anti-TNF agents continues to increase, due to the complications and treatment failures associated with disease-modifying anti-rheumatic drugs (DMARDs). However, it remains unclear whether anti-TNF agents are beneficial or detrimental to ILD patients; therefore, additional research is required.
Hagiwara et al. [
6] reviewed a nine-case series in which RA-ILD developed following anti-TNF agent use: old age and preexisting ILD were risk factors for mortality in anti-TNF agent-induced ILD, which tends to occur shortly after treatment. The present, retrospective study determined the effects of anti-TNF treatment on mortality in RA-ILD patients. Preexisting ILD was documented in 23 of 24 patients (96%), of whom six died (26%). The majority of the deaths occurred in patients Ōēź 70 years of age, and within several months of initiating anti-TNF therapy. Although the OR, of age at anti-TNF therapy initiation for death, was not significant, our data indicates the possibility of severe complications in RA-ILD patients treated with anti-TNF agents.
The British Society for Rheumatology Biologics Register reported that RA-ILD mortality rate was not increased following treatment with anti-TNF therapy versus traditional DMARDs, but the proportion of deaths attributable to RA-ILD was higher in patients treated with anti-TNF agents [
10]. In our study, five of six deaths (83%) were possibly related to lung complications; two patients died of diffuse alveolar hemorrhage, two of acute exacerbation of ILD, and one of pneumonia.
We also identified two diffuse alveolar hemorrhage cases, similar to previous reports [
11,
12]. Although the mechanism by which anti-TNF precipitates diffuse alveolar hemorrhage remains unclear, we speculate that hemorrhage of the lung may be particularly important.
In three patients Ōēź 70 years of age, the condition of whom failed to improve despite anti-TNF therapy (and who were subsequently treated with rituximab), attenuated RA progression without lung complications was observed. Rituximab can also induce radiographic stabilization and improved lung function in systemic sclerosis-associated ILD, suggesting a beneficial effect on B-cell depletion [
13,
14]. Further research is required to determine the safety of rituximab for RA-ILD patients.
Our study had several limitations: first, RA-ILD and fibrosis status differed among patients at anti-TNF therapy initiation, and older RA-ILD patients tended to be treated with etanercept to avoid possible infection.
In conclusion, anti-TNF agents should be prescribed cautiously in older RA patients predisposed to ILD, and physicians should be aware of the potential for lung-related complications in the first year of anti-TNF therapy.
KEY MESSAGE
The complications associated with anti-tumor necrosis factor (anti-TNF) therapy for rheumatoid arthritis-associated interstitial lung disease (RA-ILD) patients are still not well-established despite several randomized controlled trials.
Of 24 RA-ILD patients receiving anti-TNF therapy, six died (25%); their age at ILD diagnosis and anti-TNF therapy initiation was Ōēź 64 years.
Physicians should be aware of the potential for lung-related complications during the first year of anti-TNF therapy.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
References
1. Tanoue LT. Pulmonary manifestations of rheumatoid arthritis. Clin Chest Med 1998;19:667ŌĆō685PMID : 9917959.


2. Suzuki A, Ohosone Y, Obana M, et al. Cause of death in 81 autopsied patients with rheumatoid arthritis. J Rheumatol 1994;21:33ŌĆō36PMID : 8151583.

4. Scott DL, Kingsley GH. Tumor necrosis factor inhibitors for rheumatoid arthritis. N Engl J Med 2006;355:704ŌĆō712PMID : 16914706.


5. Vassallo R, Matteson E, Thomas CF Jr. Clinical response of rheumatoid arthritis-associated pulmonary fibrosis to tumor necrosis factor-alpha inhibition. Chest 2002;122:1093ŌĆō1096PMID : 12226061.


6. Hagiwara K, Sato T, Takagi-Kobayashi S, Hasegawa S, Shigihara N, Akiyama O. Acute exacerbation of preexisting interstitial lung disease after administration of etanercept for rheumatoid arthritis. J Rheumatol 2007;34:1151ŌĆō1154PMID : 17444583.

7. Antoniou KM, Mamoulaki M, Malagari K, et al. Infliximab therapy in pulmonary fibrosis associated with collagen vascular disease. Clin Exp Rheumatol 2007;25:23ŌĆō28PMID : 17417986.

9. Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315ŌĆō324PMID : 3358796.


11. Khaja M, Menon L, Niazi M, Fuentes GD. Diffuse alveolar hemorrhage and acute respiratory distress syndrome during treatment of rheumatoid arthritis with etanercept. J Bronchology Interv Pulmonol 2012;19:228ŌĆō231PMID : 23207468.


12. Panagi S, Palka W, Korelitz BI, Taskin M, Lessnau KD. Diffuse alveolar hemorrhage after infliximab treatment of Crohn's disease. Inflamm Bowel Dis 2004;10:274ŌĆō277PMID : 15290924.


14. Lafyatis R, O'Hara C, Feghali-Bostwick CA, Matteson E. B cell infiltration in systemic sclerosis-associated interstitial lung disease. Arthritis Rheum 2007;56:3167ŌĆō3168PMID : 17763433.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





