 |
 |
| Korean J Intern Med > Volume 30(4); 2015 > Article |
|
Abstract
The prevalence of hepatitis C virus (HCV) in Asia is 0.5% to 4.7%, with three different genotypes predominating, depending on the geographic region: genotype 1b in East Asia, genotype 3 in South and Southeast Asia, and genotype 6 in Indochina. Official approval for direct-acting antiviral agents (DAAs) in Asia lags significantly behind that in the West, such that in most countries the mainstay of therapy is still pegylated interferon and ribavirin (PR). Because the interleukin-28B genetic variant, associated with a high sustained virologic response (SVR), is common in Asians, this treatment is still acceptable in Asian patients with HCV infections. A roadmap for HCV therapy that starts with PR and takes into account those DAAs already approved in some Asian countries can provide guidance as to the best strategies for management, particularly of genotype 1 and 3 infections, based on SVR rates. Sofosbuvir and PR are likely to be the initial therapies for genotype 1 and 3 disease, although in the former these drugs may be suboptimal in patients with cirrhosis (62% SVR) and the extension of treatment to 24 weeks may be required. For difficult to treat genotype 3 infections in treatment-experienced patients with cirrhosis, a combination of sofosbuvir and PR result in an 83% SVR and is, therefore, currently the optimal treatment regimen. Treatment failure is best avoided since data on rescue therapies for DAA failure are still incomplete.
Worldwide, over 170 million people may be infected with hepatitis C virus (HCV) [1], with > 50% living in Asia and an estimated burden of 124 million persons [2]. In the recent Global Burden of Disease Survey [3], viral hepatitis accounted for over 1 million deaths in Asia, of which ~20% were due to chronic hepatitis C. This large burden of disease is compounded by a number of issues, such as inadequate data on disease prevalence, poor screening programs, lack of infrastructure, insufficient number of trained healthcare personnel, policy inaction, delayed access to healthcare, and the low priority given to HCV infections in healthcare budgets. Asia is made up of a wide variety of nations in which the gross domestic product (GDP) ranges from very low (Bangladesh GDP per capita $2,853) to very high (Macau $138,025) and where the quality and availability of healthcare differ greatly. Hepatitis C disease, like many infectious diseases, is closely linked to a country's socio-economic status. Because many Asian countries still occupy the lower rankings of countries based on GDP [4], their healthcare budgets are under duress and priority for the treatment of viral hepatitis is low. Consequently, the HCV problem in Asia is a complex one and each country has specific issues that need to be addressed before it can be resolved.
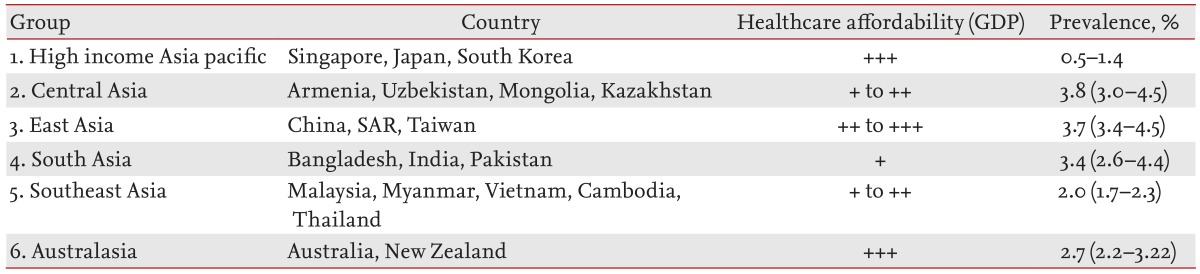
Given the variability and multitude of countries in Asia, the Global Burden of Disease Study [5] grouped Asian countries based on epidemiological homogeneity, so as to allow meaningful healthcare conclusions to be drawn for countries clustered in the same group (Table 1). However, Asian countries can also be assessed based on their healthcare reimbursement patterns; either full or almost full reimbursement, partial reimbursement, or no reimbursement [6]. Healthcare systems in which reimbursement is based on a single payer will have a stronger negotiating position with pharmaceutical companies regarding drug costs. In the absence of a single payer, the cost of drugs maybe set by the pharmaceutical company based on market forces.
A recent systematic review of the epidemiology of HCV in Asia and Australia [7] showed that even within the same country, there may be high discordance in HCV prevalence rates between studies, due to the multiethnicity and socioeconomic heterogeneity within the same country. A mathematical prediction model [2] identified the countries in Central, South, and East Asia (Mongolia, China, Taiwan, and Pakistan) as those where HCV prevalence rates exceed 3%. Pakistan (4.7%) [8] and Taiwan (4.4%) [9] had the highest prevalence, followed by Australia, New Zealand, and the Southeast Asian countries (2% to 3%). As expected, in the high-income countries of Japan, South Korea, and Singapore, where community hygiene standards are the highest in Asia, HCV prevalence is the lowest [2].
The diversity of HCV genotypes in Asia [7] is related to the highly diverse ethnicity and routes of transmission in Asia Pacific countries. Genotype 1b is the predominant genotype (45% to 64%) in East Asian countries (China, Taiwan, South Korea, and Japan), followed by genotype 2 infection. Australia has a mix of genotypes 1 and 3 (54% and 37%, respectively). In South and Southeast Asia, genotype 3 (45% to 79%) predominates in Thailand, India, and Pakistan. Interestingly, Vietnam has a high predominance of genotypes 1 and 6 (30%, 54%), a pattern that is not found elsewhere in Asia. The differences in genotype distribution have significant implications for Asian countries. Infections with genotype 3, prevalent in South Asia and Southeast Asia, are difficult to treat and are associated with a worse prognosis [10]. The therapeutic options for genotype 3 are limited since most of the new oral direct-acting antiviral agents (DAAs) are active against genotype 1 whereas their activity against genotype 3 is limited [10]. In Indochina, where genotype 6 is the dominant genotype, there are relatively few studies on the efficacy of antiviral agents. These challenges make broad recommendations and guidelines of limited value in these countries.
The efficacy of DAAs in different HCV populations has been updated in the most recent European Association for the Study of the Liver (EASL) clinical practice guidelines [11]. The 2015 EASL guidelines allow for the use of pegylated interferon and ribavirin (PR) in situations in which no other treatment options are available, which is exactly the scenario in Asia. However, this therapeutic strategy is not recommended by the American Association for the Study of Liver Diseases (AASLD) guidelines [12]. The Asian Pacific Association for the Study of the Liver (APASL) guidelines [13], published in 2012, have not been updated since that date and, thus, do not take into account the new DAAs.
Meta-analyses have shown that the pooled prevalence of the favorable interleukin-28B interferon response genotype is more common in Asians (73%) than in Caucasians (41%) and African-Americans (13%) [14]. This is thought to account for the high sustained virologic response (SVR) rates (76%) achieved in Asian patients after 48 weeks of PR therapy [15] and has allowed a shortened 24 weeks of treatment in patients with other predictors of a good response [16]. Nonetheless, PR therapy is not an attractive choice due to its adverse event profile, high rate of discontinuation, and the need for close monitoring and trained medical personnel. It is therefore unlikely to be an effective strategy for the widespread eradication of HCV. However, it is an inexpensive option in many Asian countries. This is particularly the case in middle-income countries, where the new DAAs are likely to be relatively costly. In low-income countries, where the cost of the DAA sofosbuvir per treatment has fallen below that of PR, the cost per SVR will be attractively priced for the new DAAs. Consequently, in some Asian countries PR may become the standard of care whereas, for cost reasons, the new DAAs maybe used as second-line or rescue therapy.
However, although PR therapy can lead to high SVR rates in Asians, selecting the appropriate treatment strategy is often difficult and there is still a lack of clarity on the optimal form of therapy. A roadmap can provide therapeutic guidance, especially with respect to the continued use of PR to treat HCV patients [17]. In brief, the strongest predictor of SVR is the HCV RNA status at week 4 of PR therapy. Undetectable HCV RNA levels at week 4 lead to a 98% SVR within 48 weeks of treatment, while detectable HCV RNA levels reduce the SVR rate to 43%, which can be improved to 53% if treatment is extended to 72 weeks. This is clearly not an attractive therapeutic option for many patients but, in the absence of alternatives, it is their only option to achieve a SVR. An important consideration is that the patients who most need treatment are typically those with advanced hepatic fibrosis or cirrhosis and are therefore most likely to also have adverse conditions such as anemia, neutropenia, thrombocytopenia, as well as clinical complications of nausea, anorexia, weight loss, and severe lethargy-all of which have been well documented in HCV-infected patients [18]. The supportive care needed for these patients includes erythropoietin, granulocyte-colony stimulating factor, and eltrombopag, which will help to avoid PR dose reductions and, therefore, an interruption in therapy leading to a lower SVR.
The first-wave DAAs of telaprevir and boceprevir had relatively little impact in Asia, because their late registration led to the deferment of therapy for eligible patients due to the impending approval of sofosbuvir (and PR), which is both more efficacious and simpler to use. Telaprevir was registered only in Japan, and boceprevir in a few Asian countries. Thus, there are no studies of the efficacy of boceprevir triple therapy in Asians except one that examined an early access program of the most difficult to treat patients; that is, those who had failed therapy and had advanced fibrosis or cirrhosis [17]. The overall SVR at 12 week posttreatment (SVR12) was 61% and was similar in Asian and Caucasian patients. Although no treatment-related deaths occurred during the study, the occurrence of significant serious adverse events and adverse events led to treatment discontinuation in many patients.
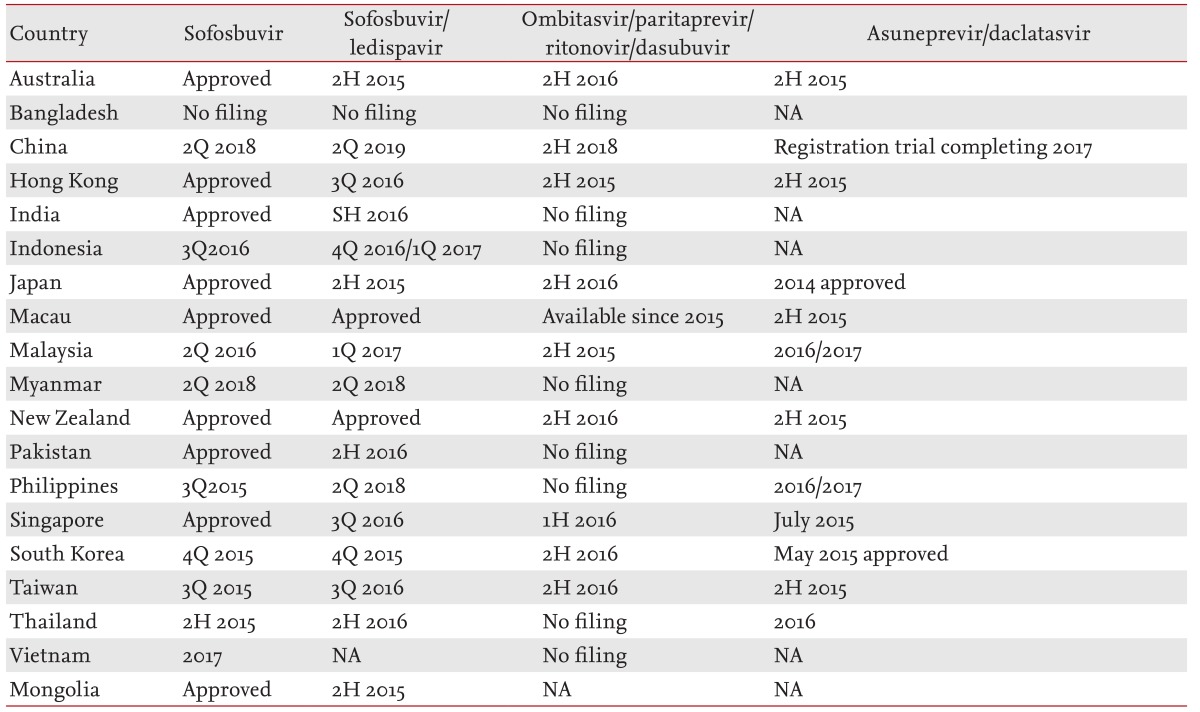
The second-wave DAAs are still used as the backbone to interferon treatment. Simeprevir is approved only in Japan and Australia. In Japan, the reduced dose of 100 mg simeprevir daily together with PR for 24 weeks has resulted in SVR rates of 88.6% in treatment-naïve patients with genotype 1 infections [19], which is similar to the rates of 80% reported in Western studies of patients administered 150 mg of simeprevir daily together with PR. The approval of sofosbuvir for use together with PR was widely anticipated, particularly in Asia, but Asian approval of the drug has been very slow (Table 2). Moreover, the efficacy of this combination in the difficult to treat, treatment-experienced population of patients with cirrhosis is unclear. This is particularly important because for genotype 1 infections the efficacy in treatment-naïve patients with versus without cirrhosis is suboptimal (80% vs. 92%) [20]. Recent, real-life management data reported by the HCV "TRIO" consortium [21] show that this combination is suboptimal in treatment-experienced patients with cirrhosis, based on a SVR rate of 62%, and in patients with genotype 1a infections and cirrhosis, in whom the SVR rate after 12 weeks of sofosbuvir and PR was 67%. This poses a conundrum for physicians in Asian countries, where, at least in the immediate future, sofosbuvir and PR are likely to be the leading therapeutic regimen for patients with genotype 1 infections. Moreover, there is no obvious alternative if this treatment fails. In this difficult to treat population, an untested strategy of sofosbuvir and PR treatment extension to 24 weeks may be a practical option and will likely yield higher SVR rates. This strategy needs to be tested either in clinical trials or real-life settings, but it is most suitable for countries where generic sofosbuvir can be purchased at a low cost, making this regimen reasonably cost effective.
One of the few all-oral DAA combinations to be tested in Asia was that of asuneprevir and daclatasvir. This combination has been approved in Japan but only for genotype 1b, by far the most common genotype 1 subtype in Asia. In a phase 3 international study (HALLMARK DUAL study) [22], in which genotype 1b patients were treated with asunaprevir and daclatasvir for 12 or 24 weeks, the SVR12 was 90% in the treatment-naive cohort, 82% in the non-responder cohort, and 82% in the ineligible, intolerant, or ineligible and intolerant cohort. In an open-label study of 222 genotype 1b patients (135 interferon-ineligible/intolerant and 87 non-responders) performed in Japan [23], a SVR24 was achieved in 87.4% of the interferon-ineligible/intolerant patients and 80.5% of non-responder (null and partial) patients. The rates were similar in patients with (90.9%) and without (84.0%) cirrhosis. Asunaprevir and daclatasvir were approved in Japan in July 2014 for the treatment of genotype 1b chronic hepatitis C. This was the first all-oral combination of DAAs to achieve initial global registration in Asia [24] and it was largely due to the high efficacy of the combination in the treatment of patients with genotype 1b infections, which, as noted above predominates among HCV infections in Japan. The efficacy of this drug combination in genotype 1a is substantially lower and the infections in these patients tend to become drug-resistant due to the development of viral mutations [25]. Nonetheless, trial data showed that in treatment-experienced patients with genotype 1b and cirrhosis the efficacy of these drugs may be considered as an alternative to sofosbuvir and PR.
The combination of sofosbuvir and ledipasvir has a high efficacy in treatment-naïve patients with and without cirrhosis but it has yet to be approved in most Asian countries. However in the more difficult to treat population of treatment-experienced patients with genotype 1 and cirrhosis, 12 weeks of therapy is less optimal (84% SVR) than the recommended 24 weeks (+ ribavirin; 100% SVR) [11]. Similarly, the combination of ombitasvir, paritaprevir, ritonavir, and dasabuvir is highly effective in treatment-naïve patients with and without cirrhosis but has reduced efficacy (83% SVR) in treatment-experienced patients with genotype 1a infections and cirrhosis when given for 12 weeks rather than the recommended 24 weeks (95% SVR) [11]. Combined simeprevir and sofosbuvir is considered to be an alternative regimen but real-world studies have shown it to be suboptimal in treatment-experienced patients with cirrhosis [11]. Sofosbuvir and daclatasvir for 24 weeks has also been shown to be an effective regimen especially in treatment naïve patients achieving 100% SVR even in cirrhosis patients [26]. Recently, the ALLY-1 study showed that advanced cirrhotics treated with sofosbuvir and daclatasvir ± ribavirin for 12 weeks achieved 83% SVR [27], and using the same regimen the real life UK "EAP" program achieved 82% SVR [28]. With so many selection choices that have high SVR rates, treatment choices are likely to be made by availability and cost.
Although widely represented in Asia, genotype 2 infections do not account for a large proportion of HCV patients. Genotype 2 is classically the easiest to treat. In a meta-analysis, the SVR rate was 74% with just 24 weeks of PR therapy but 84% in patients who achieved a rapid virologic response (RVR) [26]. A shortened therapy duration of 12 to 16 weeks was also evaluated but it was associated with a lower SVR except in patients with a low viral load. The all-oral combination of sofosbuvir and ribavirin for 12 weeks results in a 95% SVR, with a better response in patients without (97%) than with (83%) cirrhosis [27]. In treatment-experienced patients, the SVR achieved with sofosbuvir and ribavirin for 12 weeks was 91% and 88% in patients without and with cirrhosis, respectively [28]. Consequently, the recommendation for the latter group was to increase treatment duration to 16 to 20 weeks, based on a higher SVR in patients treated with sofosbuvir and ribavirin for 16 rather than 12 weeks [29]. However, in another study, the administration of sofosbuvir and PR to treatment-experienced patients yielded a SVR rate of 96% in patients without and 100% in those with cirrhosis [30]. In treatment-experienced patients, the SVR achieved with sofosbuvir and ribavirin for 12 weeks was 91% and 88% in patients without and with cirrhosis, respectively [31]. Consequently, the recommendation for the latter group was to increase treatment duration to 16 to 20 weeks, based on a higher SVR in patients treated with sofosbuvir and ribavirin for 16 rather than 12 weeks [32]. However, in another study, the administration of sofosbuvir and PR to treatment-experienced patients yielded a SVR rate of 96% in patients without and 100% in those with cirrhosis [33]. In Asia, the high efficacy of PR and its low cost will make this an attractive option, except in countries where generic sofosbuvir-whether in combination with ribavirin for 12 to 16 weeks or in combination with PR for 12 weeks-is even more cost effective than PR therapy.
Infections with genotype 3 pose a major problem in South and Southeast Asia, where it is the dominant genotype in some countries, including Pakistan. Treatment with PR for 24 weeks is the standard of care in most Asian countries. In a meta-analysis, the overall SVR was 68%, but in patients who achieve a RVR it was 86% [29]. Although treatment extension to 48 weeks is possible for patients with detectable HCV RNA at week 4, the SVR rates are still suboptimal after 48 weeks of treatment, based on an intent-to-treat analysis (61% for 48 weeks vs. 52% for 24 weeks, p = 0.1934). Nonetheless, patients who completed treatment appeared to benefit, based on a 73% SVR after 48 weeks therapy compared to 54% SVR after 24 weeks therapy (Fig. 2) [34]. In patients with cirrhosis, treatment extension to 48 weeks resulted in a slightly lower SVR than that achieved after 24 weeks (40% vs. 46%) [35].
Among the new DAAs, sofosbuvir and PR for 12 weeks or sofosbuvir and ribavirin for 24 weeks are emerging as the therapy of choice in countries where these drugs are available. In a small, open-label study, the SVR in treatment-experienced patients with and without cirrhosis was 83% [33]. The interferon-free combination of sofosbuvir and ribavirin for 24 weeks appears to have a high efficacy in treatment-naïve patients without (SVR 95%) or with (SVR 92%) cirrhosis but in treatment-experienced patients the efficacy was lower (87% SVR) especially in patients with cirrhosis (62%) [31]. Hence, this regimen was not recommended by the most recent EASL guidelines [11], but it may still be useful in Asia for treatment-naïve patients without or with cirrhosis who are either interferon ineligible or intolerant. It is a particularly attractive option in countries where, because of the availability of generic sofosbuvir, this regimen is an affordable one. The efficacy of a combination of sofosbuvir and daclatasvir for 12 weeks against genotype 3 was recently demonstrated, but the SVR results were better in patients, both treatment-naïve and treatment-experienced, without than with cirrhosis (95% and 91% vs. 73% and 63%, respectively) [36]. In the French Multicentre Compassionate program, real life data showed that in cirrhotic patients (most were treatment experienced) 12 weeks of sofosbuvir and daclatasvir ± ribavirin achieved 76% SVR compared to 24 weeks treatment achieving 88% SVR [37]. This regimen appears to be the best all oral therapy for genotype 3 treatment experienced cirrhotics. However, it does not appear to be vastly superior to 12 weeks of sofosbuvir and PR, which has a good efficacy (83%) even in the most difficult to treat group of treatment-experienced patients with cirrhosis.
This genotype is largely confined to Indochina and the immediate surrounding regions. The standard of care has been PR. In one meta-analysis, the SVR with 48 weeks of PR was 80.2% [38] while in another it was 75% [39]. In the Neutrino study, all six patients with genotype 6 infections treated with sofosbuvir and PR achieved a SVR [30]. The all-oral combination of sofosbuvir and ledipasvir for 12 weeks yielded a 96% SVR in treatment-naïve and treatment-experienced patients with genotype 6 disease [40]. However, since the approval of this combination still lags far behind approval in the West, the use of this combination in the immediate future is unlikely. Although EASL guidelines [11] recommend sofosbuvir and daclatasvir for 12 weeks based on their activity against genotype 6, clinical data are lacking and, as in the case of genotype 3 infections, this combination is unlikely to be affordable for most patients.
Owing to the high variability in economic spending power in healthcare amongst Asian countries, their health systems are highly heterogeneous. In Asia, the GDP per capita in 70% of the countries is < 20,000 USD [4] and health expenditure as a percentage of GDP was 4.5% in 2010. This indicator varies depending on the country, ranging from 2% in Myanmar up to 10.1% in New Zealand, but almost all countries fall short of the Organisation for Economic Cooperation and Development (OECD) average of 9.5% [41]. Unlike those Western countries where universal healthcare is available, either through single-payer or comprehensive insurance, most healthcare systems in Asia are based on a hybrid system in which government subsidies are limited and the patient is the direct payer for expensive or new drugs [41]. Even in developed countries, the high cost of DAAs would overwhelm the health budgets of most nations [42]. Healthcare systems in Asia are broadly divided into those with and without reimbursement [6]. In the former (Japan, South Korea, Taiwan, and Australasia), health ministries dictate policy on which treatment strategies are reimbursable. Their decisions are generally based on cost-effectiveness analyses. In a single-payer system with a large treatment volume, the bargaining power of these ministries allows them to negotiate an attractive price. In countries without reimbursement, treatment strategies are based on drug availability, affordability, and market forces. For instance, in Australia, although sofosbuvir has been approved for the treatment of HCV, reimbursement is not currently possible. Hence, patients who are likely to benefit from sofosbuvir-based therapy will have to pay out of pocket as the first-line therapy is still simeprevir triple therapy. In Asia, only in Japan has an all-oral therapy, asunaprevir and daclatasvir, been approved for genotype 1b treatment, which means that the cost will be reimbursed. Consequently, HCV guidelines in Japan are quite different from those in the rest of Asia and in Australia.
Under the HCV treatment expansion program, the American pharmaceutical company Gilead Sciences has licensed generic pharmaceutical manufacturers to produce sofosbuvir for low-income countries based on GDP [43]. The countries in Asia that would benefit from this program are Afghanistan, Bangladesh, Bhutan, Cambodia, Indonesia, India, Mongolia, Myanmar, Central Asian countries, Pakistan, Sri Lanka, and Vietnam. Yet, despite the improved access to the drug in many of the low-income countries in Asia there are still several challenges, including the final cost of these generic versions in relation to their affordability for patients in need, the absence of the infrastructure needed to identify patients and ensure that they have access to these drugs, and mechanisms to avoid non-compliance and, thereby, the emergence of widespread resistance in the community [4445]. Furthermore, the commitment and scale of HCV programs by Asian governments pale when compared to the tremendous strides made in Egypt. National liver associations, non-governmental organisations such as the Coalition for Eradication of Viral Hepatitis in the Asia Pacific (CEVHAP) and the World Health Organization are currently mapping out strategies, but without the appropriate infrastructure and resources in each nation their impact is likely to be limited.
Due to the differences in HCV prevalence, genotype, healthcare systems, economic priorities, and infrastructure, a one size fits all strategy for HCV therapy would be doomed to failure, especially now, when therapeutic strategies are still in a state of flux and a pan-genotypic therapy has yet to be developed. The anticipated approval dates in Asia for DAAs are listed in Table 2. The all-oral DAA combination of asunaprevir and daclatasvir may be a useful therapy for patients in countries such as Japan. With the impending approval of sofosbuvir, many patients have chosen to defer therapy in order to be treated with the drug. Sofosbuvir and PR for 12 weeks is likely to be the standard of care for many patients with genotype 1 disease, but this approach is suboptimal in treatment-experienced patients with cirrhosis. In the latter group, studies of the efficacy of therapy extended to 24 weeks are needed. In middle-income countries, sofosbuvir use is likely to be high given that most Asians have genotype 1b disease. For patients with genotype 2, PR for 24 weeks leads to high SVR rates even in those with cirrhosis. Sofosbuvir and PR for 12 weeks or sofosbuvir and ribavirin for 16 weeks in patients with cirrhosis are alternatives. For treatment-experienced patients with genotype 3 and cirrhosis, the best SVR rates are achieved with sofosbuvir and PR for 12 weeks or interferon-free therapy with sofosbuvir and daclatasvir for 24 weeks. Sofosbuvir and ribavirin for 24 weeks are not recommended in the most recent EASL guidelines, although it may be a possible option for treatment naïve patients in countries that have access to generic sofosbuvir, but not for treatment experienced cirrhotics. Finally, the data on genotype 6 infections are scarce, except that the combination of sofosbuvir and PR for 12 weeks seems to be a good option in the treatment-naïve population.
A new roadmap for the management of HCV patients in Asia is proposed in Figs. 1 and 2. It may prove useful, since the last APASL guidelines were published in 2012 [13]. The roadmap provides updated information subsequent to the approval of boceprevir [46] and to the most recent EASL clinical practice guidelines [11] and guides choices based on the availability and cost of treatment. Ultimately, the decision to be made is whether PR should be used as the initial therapy. As rescue therapy for treatment-experienced patients, the results are suboptimal. Although PR is currently the only treatment option for patients in several countries, such as China, it has many well-known limitations in patients considered difficult-to-treat, such as those with advanced or decompensated cirrhosis, post liver or renal transplantation, HIV co-infection, and autoimmune disease. These patients certainly need access to all-oral DAAs.
The rapid advances in hepatitis C treatment have led to a paradigm change. HCV infections are now considered curable and can potentially be eradicated. The majority of the HCV burden is in Asia, where significant challenges exist in our attempts to eradicate this disease. We are in a dynamic transition period in which there are a multitude of competing forces. Approval rates for DAAs lag significantly behind those of Western countries but these drugs will eventually become widely available. The treatment roadmap proposed herein provides guidance for HCV management in the short to medium term. In Asia, there will be widely differing prices for DAAs due to the presence of access programs in low-GDP countries and commercial interests in middle- to high-income countries. This differential in pricing is already breeding medical tourism, as patients seek alternatives to access affordable treatments. Current treatment regimens are in a state of flux, as studies for a pan-genotypic strategy and to reduce treatment duration are still in progress. However, the larger problems in Asia, i.e., the large burden of undiagnosed disease and the lack of adequate screening programs, have yet to be tackled.
1. More than 50% of hepatitis C virus (HCV) carriers worldwide reside in Asia but the prevalence is heterogeneous, with pockets of high and low prevalence.
2. The HCV genotype distribution varies by region. In East Asia, genotype 1b predominates, while in South and Southeast Asia, genotype 3 is the dominant form, and in Indochina genotype 6.
3. The Global Burden of Disease Survey has divided Asia into six regions based on epidemiological similarities
4. Asians have a much higher prevalence of the favorable genetic variant interleukin-28B, which is associated with a high sustained virologic response (SVR) rate (> 70%).
5. The registration approval of new direct acting antivirals (DAAs) in Asia lags behind that of Western countries, with delays as long as 3 to 4 years.
6. Due to the diversity in epidemiology and viral genotype distribution, healthcare systems, economic status, reimbursement policies, and access to new DAAs, a one size fits all strategy is unlikely to succeed with the current treatment strategies.
7. A roadmap for HCV management based on available therapies and their SVR rates can guide treatment selection.
8. Generic licensing and access programs in low-income countries will make DAAs more accessible.
9. Because screening and linkage to care are underdeveloped in Asia, it will be many decades before the burden of disease can be truly addressed.
Conflict of Interest
Conflict of interest: No potential conflict of interest relevant to this article
was reported.
References
1. Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect 2011;17:107ŌĆō115PMID : 21091831.


2. Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology 2013;57:1333ŌĆō1342PMID : 23172780.


3. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2095ŌĆō2128PMID : 23245604.


4. International Monetary Fund. Download entire World Economic Outlook database [Internet]. Washington (DC): International Monetary Fund, 2015;cited 2015 Apr 22. Available from: http://www.imf.org/external/pubs/ft/weo/2015/01/weodata/download.aspx.
5. Institute for Health Metrics and Evaluation. Global Burden Of Diseases, Injuries And Risk Factors Study Operations Manual [Internet]. Seattle (WA): Institute for Health Metrics and Evaluation, c2015;cited 2015 Apr 23. Available from: http://www.globalburden.org.
6. Lim SG, Amarapurkar DN, Chan HL, et al. Reimbursement policies in the Asia-Pacific for chronic hepatitis B. Hepatol Int 2015;9:43ŌĆō51PMID : 25788378.


7. Sievert W, Altraif I, Razavi HA, et al. A systematic review of hepatitis C virus epidemiology in Asia, Australia and Egypt. Liver Int 2011;31(Suppl 2):61ŌĆō80PMID : 21651703.


8. Umar M, Bushra HT, Ahmad M, et al. Hepatitis C in Pakistan: a review of available data. Hepat Mon 2010;10:205ŌĆō214PMID : 22308140.


9. Chen CH, Yang PM, Huang GT, Lee HS, Sung JL, Sheu JC. Estimation of seroprevalence of hepatitis B virus and hepatitis C virus in Taiwan from a large-scale survey of free hepatitis screening participants. J Formos Med Assoc 2007;106:148ŌĆō155PMID : 17339159.


10. Ampuero J, Romero-Gomez M, Reddy KR. Review article: HCV genotype 3: the new treatment challenge. Aliment Pharmacol Ther 2014;39:686ŌĆō698PMID : 24612116.


11. European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2015. J Hepatol 2015;63:199ŌĆō236PMID : 25911336.


12. AASLD/IDSA/IAS-USA. Recommendations for testing, managing, and treating hepatitis [Internet] c2014. cited 2015 May 22. Available from: http://www.hcvguidelines.org.
13. Omata M, Kanda T, Yu ML, et al. APASL consensus statements and management algorithms for hepatitis C virus infection. Hepatol Int 2012;6:409ŌĆō435.


14. Rangnekar AS, Fontana RJ. Meta-analysis: IL-28B genotype and sustained viral clearance in HCV genotype 1 patients. Aliment Pharmacol Ther 2012;36:104ŌĆō114PMID : 22612303.


15. Liu CH, Liu CJ, Lin CL, et al. Pegylated interferon-alpha-2a plus ribavirin for treatment-naive Asian patients with hepatitis C virus genotype 1 infection: a multicenter, randomized controlled trial. Clin Infect Dis 2008;47:1260ŌĆō1269PMID : 18834319.


16. Liu CH, Liang CC, Liu CJ, et al. Interleukin 28B genetic polymorphisms and viral factors help identify HCV genotype-1 patients who benefit from 24-week pegylated interferon plus ribavirin therapy. Antivir Ther 2012;17:477ŌĆō484PMID : 22301466.


17. Sukeepaisarnjaroen W, Pham T, Tanwandee T, et al. Boceprevir early-access for advanced-fibrosis/cirrhosis in Asia-pacific hepatitis C virus genotype 1 non-responders/relapsers. World J Gastroenterol 2015;1. 8. [Epub].

18. Dusheiko G. Side effects of alpha interferon in chronic hepatitis C. Hepatology 1997;26(3 Suppl 1):112SŌĆō121SPMID : 9305675.

19. Hayashi N, Izumi N, Kumada H, et al. Simeprevir with peginterferon/ribavirin for treatment-naive hepatitis C genotype 1 patients in Japan: CONCERTO-1, a phase III trial. J Hepatol 2014;61:219ŌĆō227PMID : 24727123.


20. Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med 2013;368:1878ŌĆō1887PMID : 23607594.


21. Dieterich D, Bacon BR, Flamm SL, et al. Evaluation of sofosbuvir and simeprevir-based regimens in the TRIO network: academic and community treatment of a real-world, heterogeneous population. Hepatology 2014;60(Suppl 1):220A.
22. Manns M, Pol S, Jacobson IM, et al. All-oral daclatasvir plus asunaprevir for hepatitis C virus genotype 1b: a multinational, phase 3, multicohort study. Lancet 2014;384:1597ŌĆō1605PMID : 25078304.


23. Kumada H, Suzuki Y, Ikeda K, et al. Daclatasvir plus asunaprevir for chronic HCV genotype 1b infection. Hepatology 2014;59:2083ŌĆō2091PMID : 24604476.



24. Poole RM. Daclatasvir + asunaprevir: first global approval. Drugs 2014;74:1559ŌĆō1571PMID : 25117197.


25. Kosaka K, Imamura M, Hayes CN, et al. Emergence of resistant variants detected by ultra-deep sequencing after asunaprevir and daclatasvir combination therapy in patients infected with hepatitis C virus genotype 1. J Viral Hepat 2015;22:158ŌĆō165PMID : 24943406.


26. Sulkowski MS, Gardiner DF, Rodriguez-Torres M, et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med 2014;370:211ŌĆō221PMID : 24428467.


27. Poordad F, Schiff ER, Vierling JM, et al. Daclatasvir, sofosbuvir, and ribavirin combination for HCV patients with advanced cirrhosis or post-transplant recurrence: ALLY-1 Phase 3 Study In : Proceedings of the 50th annual Meeting of the European Association for the Study of the Liver; 2015 Apr 22-26; Vienna (AU). Geneva (CH): European Association for the Study of the Liver, 2015.
28. Foster GR, McLauchlan J, Irving W, et al. Treatment of decompensated HCV cirrhosis in patients with diverse genotypes: 12 weeks sofosbuvir and NS5A inhibitors with/without ribavirin is effective in HCV genotypes 1 and 3 In : Proceedings of the 50th annual Meeting of the European Association for the Study of the Liver; 2015 Apr 22-26; Vienna (AU). Geneva (CH): European Association for the Study of the Liver, 2015.
29. Andriulli A, Mangia A, Iacobellis A, Ippolito A, Leandro G, Zeuzem S. Meta-analysis: the outcome of anti-viral therapy in HCV genotype 2 and genotype 3 infected patients with chronic hepatitis. Aliment Pharmacol Ther 2008;28:397ŌĆō404PMID : 18549461.


30. Lawitz E, Gane EJ. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med 2013;369:678ŌĆō679PMID : 23944316.

31. Zeuzem S, Dusheiko GM, Salupere R, et al. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med 2014;370:1993ŌĆō2001PMID : 24795201.


32. Jacobson IM, Gordon SC, Kowdley KV, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med 2013;368:1867ŌĆō1877PMID : 23607593.


33. Lawitz E, Poordad F, Brainard DM, et al. Sofosbuvir with peginterferon-ribavirin for 12 weeks in previously treated patients with hepatitis C genotype 2 or 3 and cirrhosis. Hepatology 2015;61:769ŌĆō775PMID : 25322962.



34. Cheinquer H, Shiffman ML, Zeuzem S, et al. The outcome of 24 vs. 48 weeks of peginterferon alfa-2a (40KD) plus ribavirin on sustained virologic response rates in patients infected with genotype 2 or 3 hepatitis C virus who do not achieve a rapid viral response: the N-CORE study. Hepatology 2012;56(Suppl 1):156A.



35. Shoeb D, Dearden J, Weatherall A, et al. Extended duration therapy with pegylated interferon and ribavirin for patients with genotype 3 hepatitis C and advanced fibrosis: final results from the STEPS trial. J Hepatol 2014;60:699ŌĆō705PMID : 24291239.


36. Nelson DR, Cooper JN, Lalezari JP, et al. All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology 2015;61:1127ŌĆō1135PMID : 25614962.



37. Hezode C, de Ledinghen V, Fontaine H, et al. Daclatasvir plus sofosbuvir with or without ribavirin in patients with hcv genotype 3 infection: interim analysis of a French multicenter compassionate use program In : Proceedings of the 50th annual Meeting of the European Association for the Study of the Liver; 2015 Apr 22-26; Vienna (AU). Geneva (CH): European Association for the Study of the Liver, 2015.
38. Nguyen NH, McCormack SA, Vutien P, et al. Meta-analysis: superior treatment response in Asian patients with hepatitis C virus genotype 6 versus genotype 1 with pegylated interferon and ribavirin. Intervirology 2015;58:27ŌĆō34PMID : 25592813.



39. Wang X, Liu F, Wei F, Ren H, Hu H. Efficacy and safety of pegylated interferon plus ribavirin therapy for chronic hepatitis C genotype 6: a meta-analysis. PLoS One 2014;9:e100128. PMID : 24963667.



40. Gane EJ, Hyland RH, An D, et al. High efficacy of LDV/SOF regimens for 12 weeks for patients with HCV genotype 3 or 6 infection. Hepatology 2014;60(6 Suppl):1274A.
41. Tarn YH, Hu S, Kamae I, et al. Health-care systems and pharmacoeconomic research in Asia-Pacific region. Value Health 2008;11(Suppl 1):S137ŌĆōS155PMID : 18387058.


42. Hoofnagle JH, Sherker AH. Therapy for hepatitis C: the costs of success. N Engl J Med 2014;370:1552ŌĆō1553PMID : 24725236.


43. Gilead Sciences. Chronic hepatitis C treatment expansion: generic manufacturing for developing countries [Internet]. Foster City (CA): Gilead, c2009;cited 2015 Apr 22. Available from: http://www.gilead.com/~/media/Files/pdfs/other/HCVGenericAgreementFactSheet.pdf.
44. Suthar AB, Harries AD. A public health approach to hepatitis C control in low- and middle-income countries. PLoS Med 2015;12:e1001795. PMID : 25757228.



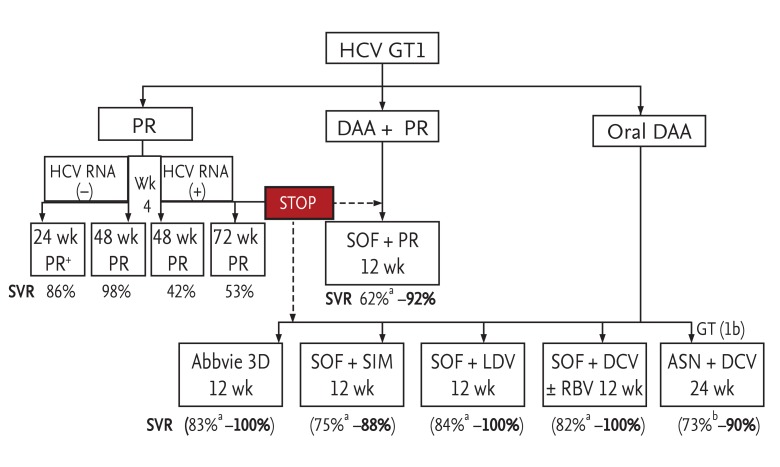
Figure┬Ā1
A 2015 roadmap for the treatment of genotype 1 (GT1) hepatitis C virus (HCV) infections in Asia. Modified from Lim [42], European Association for the Study of the Liver [11], and Manns et al. [22]. P, pegylated interferon; R, ribavirin; DAA, direct-acting antiviral; SOF, sofosbuvir; SVR, sustained virologic response; Abbvie 3D, mbitasvir, paritaprevir, ritonavir, and dasabuvir; SIM, simeprevir; LDV, ledipasvir; DCV, daclatasvir; RBV, ribavirin; ASN, asuneprevir. aFor treatment-experienced patients with cirrhosis; bFor patients with advanced cirrhosis with low platelets.
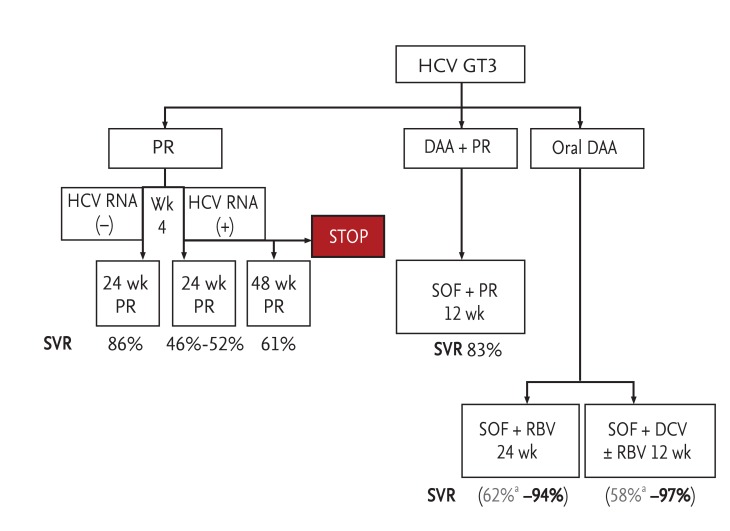
Figure┬Ā2
A 2015 roadmap for the treatment of genotype 3 (GT3) hepatitis C virus (HCV) infections in Asia. Based on European Association for the Study of the Liver [11], Andriulli et al. [26], and Cheinquer et al. [31]. P, pegylated interferon; R, ribavirin; DAA, direct-acting antiviral; SVR, sustained virologic response; SOF, sofosbuvir; DCV, daclatasvir; RBV, ribavirin. aTreatment-experienced patients with cirrhosis.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



