 |
 |
| Korean J Intern Med > Volume 30(5); 2015 > Article |
|
Abstract
Background/Aims:
Liver transplant patients are at high risk for methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci (VRE) colonization. We evaluated patients before and after liver transplant using active surveillance culture (ASC) to assess the prevalence of MRSA and VRE and to determine the effect of bacterial colonization on patient outcome.
Methods:
We performed ASC on 162 liver transplant recipients at the time of transplantation and 7 days posttransplantation to monitor the prevalence of MRSA and VRE.
Results:
A total of 142 patients had both nasal and rectal ASCs. Of these patients, MRSA was isolated from 12 (7.4%) at the time of transplantation (group 1a), 9 (6.9%) acquired MRSA posttransplantation (group 2a), and 121 did not test positive for MRSA at either time (group 3a). Among the three groups, group 1a patients had the highest frequency of developing a MRSA infection (p < 0.01); however, group 2a patients had the highest mortality rate associated with MRSA infection (p = 0.05). Of the 142 patients, VRE colonization was detected in 37 patients (22.8%) at the time of transplantation (group 1b), 21 patients (20%) acquired VRE posttransplantation (group 2b), and 84 patients did not test positive for VRE at either time (group 3b). Among these three groups, group 2b patients had the highest frequency of VRE infections (p < 0.01) and mortality (p = 0.04).
Conclusions:
Patients that acquired VRE or MRSA posttransplantation had higher mortality rates than did those who were colonized pre-transplantation or those who never acquired the pathogens. Our findings highlight the importance of preventing the acquisition of MRSA and VRE posttransplantation to reduce infections and mortality among liver transplant recipients.
Methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci (VRE) are significant causes of nosocomial infections in transplant recipients [1]. In a previous study, we reported that the prevalence of MRSA or VRE infection among liver transplant recipients was approximately 8.8% and 3.4%, respectively [2]. Liver transplant patients have a high risk of colonization of multidrug-resistant bacteria such as MRSA and VRE because of multiple hospital admissions, prolonged hospital stays, and recent use of broad-spectrum antibiotics for treatment. Several studies have demonstrated that VRE or MRSA colonization precedes active infection in individuals with various underlying diseases [3-6] and that the incidence of infection from colonization varies across patient populations. Among transplant recipients, pretransplant nasal carriage of MRSA and rectal VRE colonization is associated with a high risk of infections. Few studies have focused on the relationship between the immediate postoperative acquisitions of drug-resistant bacteria in patients residing in the intensive care unit (ICU) and the subsequent rate of bacterial infections in these patients [7-10]. In cases of limited resources, facilities rarely perform routine active surveillance cultures (ASCs) because of their high costs. However, hospitals in some countries, like Korea, perform ASCs upon initial admission to the ICU.
In this study, the hospital performed ASCs for MRSA and VRE at the time of transplantation and 7 days posttransplantation, prior to patients leaving the ICU. We evaluated the prevalence of MRSA or VRE colonization among liver transplant recipients and the rate of acquisition of these pathogens posttransplantation. Furthermore, we compared the clinical outcomes among patients who were initially colonized with MRSA and VRE prior to transplantation versus those who acquired the pathogens posttransplantation versus those who never tested positive for either pathogen during the pre- and postoperative periods.
This prospective study was conducted on inpatients from a 1,200-bed tertiary hospital. For infection control, routine ASCs have been performed in the ICU of this hospital since 2008. From January 2008 to December 2010, we performed ASCs to detect MRSA and VRE among liver transplant recipients at the time of transplantation and 7 days posttransplantation. Subjects were eligible for inclusion if they had been monitored for both MRSA and VRE.
We collected the following demographic data: comorbidities, Child-Pugh scores, Model for End-Stage Liver Disease (MELD) scores, VRE infection and source, MRSA infection and source, and clinical outcome. Infection was defined using previously reported criteria [11]. Presence of an invasive device was defined as the insertion of a device for 7 days during a hospital stay prior to transplantation. Re-transplantation was defined in patients who had undergone a second transplantation. All postoperative procedures were documented (i.e., dialysis 7 days prior to transplantation). For all liver transplant patients who met our abovementioned criteria, we determined the incidence of colonization and acquisition of MRSA and VRE, the risk factors for infection, and the clinical outcomes.
This study was approved by Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Investigational Review Board (KC12RISI0914).
Perioperative prophylaxis consisted of a 5-day regimen of intravenous injections of cefoperazone/sulbactam (2 g/day) and ampicillin (8 g/day). For fungal prophylaxis, nystatin (800,000 units/day) was administered orally for 1 month. Pneumocystis pneumonia prophylaxis consisted of oral administration of trimethoprim/sulfamethoxazole (80 mg/400 mg/day) for 3 months. Routine antiviral prophylaxis was not administered.
All patients received tacrolimus or cyclosporine, mycophenolate mofetil, and low-dose prednisone as routine immunosuppressive agents.
Perianal swabs were cultured for VRE by inoculation into Enterococcosel broth supplemented with 6 ╬╝g/mL vancomycin (Becton Dickinson, Franklin Lakes, NJ, USA) and incubation at 35┬░C for 18 to 24 hours. Positive-VRE samples were gram-stained, plated onto blood agar, and incubated at 35┬░C for 18 to 24 hours. Testing for pyrrolidonyl arylamidase activity (Oxoid, Cambridge, UK) was used to identify enterococci [12]. Susceptibility testing for vancomycin was performed using a disk-diffusion method. Minimum inhibitory concentrations for vancomycin and teicoplanin were determined following the Clinical and Laboratory Standards Institute guidelines [13]. Enterococcus faecium ATCC 29212 was used as a reference strain. Multiplex polymerase chain reaction (PCR) and the Seeplex VRE ACE detection kit (Seegene Inc., Seoul, Korea) were utilized to identify all Enterococcus species [14].
To detect MRSA, ASC was obtained from the anterior nares of patients and inoculated in Amies medium (Micromedia Co., Seoul, Korea). Subcultures were inoculated onto Brilliance MRSA II Agar (Oxoid) and read following an 18 to 24 hours incubation at 37┬░C. Staphylococcus aureus was identified by positive tests for catalase or coagulase production.
The infection control policy of our hospital mandates that all doctors, house staff, and nurses in the ICU wash their hands. Contact precautions were taken for known cases of MRSA or VRE colonization and infection. Eradication therapy such as intranasal mupirocin is not performed routinely. In cases with VRE colonization and infection, patients were grouped.
Student t test and the Mann-Whitney U test were used to analyze continuous variables. The chi-square test and Fisher exact test were used to analyze categorical variables. We analyzed risk factors associated with MRSA/VRE acquisition in patients not initially colonized with these pathogens using univariate analysis. Variables with a p < 0.1 in the univariate analysis were added in a forward stepwise manner and selected to create the final model for the multivariate analysis. Variables were included as described previously [5,10,15]. Data were analyzed using SPSS version 13.0 (SPSS Inc., Chicago, IL, USA), and a p < 0.05 was considered statistically significant.
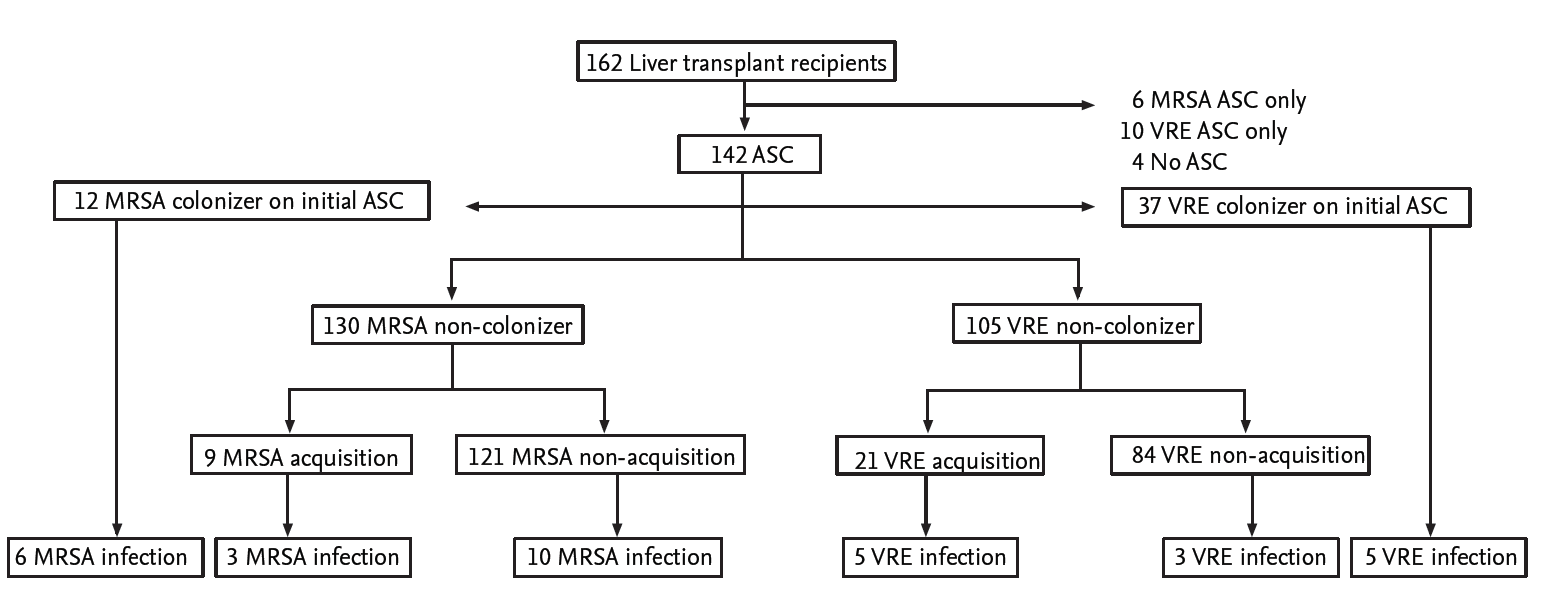
Among 162 liver transplant recipients, 142 patients (87.7%) had both nasal and rectal ASCs at the time of transplantation. The median age was 50 years (interquartile range, 41 to 56), and 65.5% were male. The most common underlying liver diseases were hepatitis B (n = 75, 52.8%), followed by alcoholic liver disease (n = 19, 13.4%), and acute hepatitis A (n = 16, 11.3%). Table 1 shows the demographics for all 142 patients. The detail of patients is displayed in Fig. 1.
MRSA nasal colonization was detected in 12 patients (7.4%) from the initial ASC. During this study, 9 of the 130 patients (6.9%) who were not initially colonized with MRSA had acquired MRSA posttransplantation.
Table 2 shows the comparisons between patients initially colonized with MRSA versus those who were not colonized. It is important to note that the preoperative hospital stay was significantly longer for patients initially colonized by MRSA than those who were not colonized (18 days vs. 4 days; p = 0.02).
Among the patients who were not initially colonized with MRSA (n = 130), we compared the baseline and clinical characteristics between those who acquired MRSA posttransplantation and those who did not (Table 2). Patients who acquired MRSA received significantly more pretransplant dialysis (55.6% vs. 14.9%; p < 0.01), were more likely to undergo retransplantation (22.2% vs. 1.7%, p < 0.01), and were more likely to undergo insertion of a hemodialysis catheter compared to those that did not (44.4% vs. 15.7%, p = 0.03). MELD scores from patients with MRSA were higher than in those who were not colonized with MRSA (26 vs. 16, p = 0.05). There was a significant difference in the presence of hepatitis A in patients who were not colonized with MRSA compared with those who acquired MRSA posttransplantation (11.6% vs. 0%, p = 0.04). Furthermore, hospital stay in the ICU was more prevalent in patients who acquired MRSA than in those who did not (11.1% vs. 1.7%).
Table 3 lists the comparisons of the clinical outcomes among all patients. In patients initially colonized with MRSA, six (50%) acquired MRSA infections including two cases of pneumonia, one of peritonitis, one catheter-related infection, and one wound infection. Among the nine patients who acquired MRSA posttransplantation, three (33.3%) developed pneumonia due to MRSA infection. Of the 121 patients who were not colonized with MRSA, 10 (8.3%) developed MRSA infections, including two pneumonia cases, four peritonitis cases, two catheter-related infections, one wound infection, and one primary bacteremia case. Liver transplant recipients initially colonized with MRSA had the highest incidence of MRSA infection posttransplantation (p < 0.01). In addition, patients who acquired MRSA posttransplantation had an overall higher mortality rate (p = 0.05) and infection-related mortality rate (p = 0.06) compared with patients initially colonized or never colonized with MRSA. There were no differences among patients in terms of the rate of transplant rejection (p = 0.27), length of postoperative hospital stay (p = 0.11), or median time to acquiring a MRSA infection posttransplantation (p = 0.14).
Based on univariate analyses, the risk factors for MRSA acquisition posttransplantation included retransplantation (odds ratio [OR], 16.6; 95% confidence interval [CI], 2.0 to 135.7; p < 0.01), MELD score (OR, 1.1; 95% CI, 1.01 to 1.13; p = 0.03), and pretransplant dialysis (OR, 7.16; 95% CI, 1.75 to 29.21; p < 0.01) (Table 4). According to multivariate analyses, retransplantation was an independent risk factor for MRSA acquisition posttransplantation (OR, 13.5; 95% CI, 1.2 to 155.2; p = 0.03).
VRE colonization was detected in 37 patients (22.8%) on initial the ASC (Table 5). Among the 105 patients not initially colonized with VRE, 21 (20%) acquired VRE posttransplantation.
Patients initially colonized with VRE had a significantly longer preoperative hospital stay (11 days vs. 4 days, p = 0.002) and higher MELD score (22 vs. 16, p = 0.02) compared with those patients not initially colonized with VRE. Among the patients (n = 105) who were not initially colonized with VRE, we compared baseline and clinical characteristics between patients who did versus did not acquire VRE posttransplantation (Table 5). Patients who acquired VRE experienced longer stays in the ICU (9 days vs. 4 days, p = 0.05) compared with those who did not.
Five patients (13.5%) initially colonized by VRE developed cholangitis and peritonitis. Among the 21 patients who acquired VRE posttransplantation, five (23.8%) developed a VRE infection (two cholangitis and three peritonitis cases). Of the 84 patients who were not colonized with VRE, three (3.6%) developed a VRE infection (two cholangitis and one urinary tract infection case). Liver transplant recipients who acquired VRE posttransplantation were more likely to develop VRE infections compared with those who were preoperatively colonized with VRE or were not colonized (p < 0.01). The mortality rate of patients who acquired VRE posttransplantation was 33.3% compared with 15.5% mortality in patients who were not colonized with VRE and 8.1% in patients preoperatively colonized with VRE. The differences in mortality rates among the groups of patients was statistically significant (p = 0.04). Transplant patients who acquired VRE had a higher infection-related mortality (n = 4, 19%) compared with those who were not colonized with VRE (n = 8, 9.5%) or those preoperatively colonized with VRE (n = 2, 5.4%). However, these differences were not statistically significant (p = 0.24). Rejection was also not significantly different among the three patient groups (Table 6).
Univariate analysis indicated that the risk factors for VRE acquisition posttransplantation were the length of ICU stay (OR, 1.07; 95% CI, 1.01 to 1.15; p = 0.05) and the length of postoperative hospital stay (OR, 1.03; 95% CI, 1.002 to 1.06; p = 0.04). However, these were not statistically significant as indicated by the multivariate analysis (Table 7).
In this study, we observed VRE colonization in 22.8% of liver transplant recipients at the time of transplantation and an acquisition of VRE in 20% of patients posttransplantation. In a previously published report, we observed VRE colonization in 17.6% of the patients admitted to our medical ICU, the same facility evaluated in this study [5]. Liver transplant recipients had higher rates of VRE colonization than those of other ICU inpatients. Initially, MRSA was detected in 7.4% of patients; however, 6.9% of patients acquired MRSA posttransplantation. Colonization and acquisition rates from this study were lower than those previously reported [10,16]. The difference may be attributed to infection control compliance among the medical team as well as the length of the follow-up conducted in this study, which may not have been long enough to detect MRSA acquisition. For example, Santoro-Lopes et al. [17] reported that the median time for MRSA acquisition was 24 days after liver transplantation. Also, there could be differences in the accuracy of MRSA detection based on the type of technique utilized (i.e., PCR and chromogenic agar), which ultimately could impact the colonization and infection rates observed [18].
This study demonstrated that patients with MRSA colonization at the time of transplantation had the highest rate of developing infections but not the highest mortality rates. Patients who acquired MRSA posttransplantation had a high rate of MRSA infection and a less favorable outcome compared with those who were not colonized. The sample size in our study was not sufficient to make definitive conclusions; however, among the patients who acquired MRSA posttransplantation were those with fulminant hepatitis and a frequency of retransplantation. These results suggest that underlying liver conditions in patients prior to live transplantation may affect MRSA acquisition posttransplantation and explain the high mortality rate associated with the acquisition of MRSA. Previously, we reported that 47 of 208 (13.3%) liver transplant recipients acquired a MRSA infection posttransplantation [2]. In this study, the rate of MRSA infection was approximately 33.3% to 50%, particularly in those patients colonized with MRSA at the time of transplantation and those who acquired MRSA posttransplantation. The high rate of MRSA infections we observed among patients is similar to the rates reported in previous studies [9,19].
Previous studies have indicated that the risk factors for MRSA colonization include severity of patients, prior antimicrobial use, presence of invasive medical devices, and prolonged ICU stays [20,21]. In this study, the factors associated with acquiring MRSA posttransplantation were underlying liver disease, MELD score, pretransplant dialysis, and retransplantation, although only retransplantation was statistically significant according to the multivariate analyses. Invasive procedures such as pretransplant dialysis and retransplantation result in longer hospital stays. Furthermore, a high MELD score or retransplantation may be indicative of a severe condition requiring more intensive care. Both scenarios increase the likelihood of MRSA transmission.
The risk of VRE infection among liver transplant recipients increased in patients who acquired rectal VRE during posttransplantation. These patients had high mortality rates. Patients colonized with VRE initially also developed a greater number of VRE infections compared with those who were not colonized. In this study, longer ICU stay was an independent risk factor for VRE acquisition among liver transplant recipients, whereas severity of patients, reflected by the Child-Pugh score and MELD score, was not.
This study has several limitations. First, we observed a small number of patients at a single facility. Second, follow-up ASC was performed during the ICU stay, which may have been too short a period to detect the acquisition of MRSA or VRE. However, transplant recipients have a high risk of colonization with multidrug-resistant bacteria due to frequent stays in the ICU. Hospitals in Korea are obligated to perform repeat ASCs for every patient because of financial insurance policies. Our study highlighted the likelihood of MRSA or VRE acquisition during an ICU stay, reflecting a typical hospital experience in Korea. Third, we did not perform genotyping using pulsed-field gel electrophoresis analysis; therefore, we could not verify that isolates from the rectal or nasal swabs were identical to those cultured from infectious foci. Lastly, measures of infection control have also been associated with acquisition of multidrug resistance organisms; however, our study were not analyzed this factor.
In summary, liver transplant recipients have a higher rate of colonization and acquisition of multidrug-resistant bacteria including MRSA and VRE. As a result, patients who acquire MRSA and VRE during transplantation have higher rates of infection and mortality. These findings highlight the importance of the preventative efforts needed to reduce acquisition of MRSA and VRE among liver transplant recipients.
1. Liver transplant recipients have higher colonization and acquisition rates for multidrug-resistant bacteria such as methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci (VRE).
2. Patients with acquired MRSA and VRE during the immediate transplant periods have higher infection and mortality rates.
Acknowledgments
We acknowledge the contributions from all intensive care unit faculty as well as the Infection Control Team of Seoul St. Mary's Hospital.
Figure┬Ā1.
Study profile. MRSA, methicillin-resistant Staphylococcus aureus; ASC, active surveillance culture; VRE, vancomycin-resistant enterococci.
Table┬Ā1.
Patient demographics (n = 142)
Table┬Ā2.
Comparison of patients who were initia lly colonized with MRSA (group 1a), acquired MRSA posttransplantation (group 2a), and were not colonized with MRSA (group 3a)
| Variable |
Initially colonized |
Not initially colonized |
p valuea | |||
|---|---|---|---|---|---|---|
| Group 1a (n = 12) | Total (n = 130) | Group 2a (n = 9) | Group 3a (n = 121) | p valueb | ||
| Age, yr | 53 (40ŌĆō61) | 49 (41ŌĆō56) | 47 (42.5ŌĆō54.5) | 50 (40ŌĆō56) | 0.94 | 0.53 |
| Male sex | 8 (66.7) | 84 (64.6) | 7 (77.8) | 77 (63.6) | 0.39 | 0.47 |
| Underlying liver disease | 0.04 | 0.58 | ||||
| ŌĆāHepatitis B | 6 (50.0) | 69 (53.1) | 4 (44.4) | 65 (53.7) | ||
| ŌĆāHepatitis C | 3 (25.0) | 8 (6.2) | 0 | 8 (6.6) | ||
| ŌĆāHepatitis B + C | 0 | 1 (1.5) | 0 | 1 (0.8) | ||
| ŌĆāHepatitis A | 2 (16.7) | 14 (10.8) | 0 | 14 (11.6) | ||
| ŌĆāCryptogenic cirrhosis | 0 | 5 (3.8) | 0 | 5 (4.1) | ||
| ŌĆāPrimary biliary cirrhosis | 0 | 1 (0.8) | 0 | 1 (0.8) | ||
| ŌĆāAlcoholic liver disease | 1 (8.3) | 18 (13.8) | 1 (11.1) | 17 (14.0) | ||
| ŌĆāOthers | 0 | 14 (10.7) | 4 (44.4) | 10 (8.3) | ||
| Cause of transplantation | ||||||
| ŌĆāDecompensated liver cirrhosis | 7 (58.3) | 72 (55.4) | 4 (44.4) | 68 (56.2) | 0.49 | 0.84 |
| ŌĆāFulminant hepatitis | 2 (16.7) | 24 (18.5) | 3 (33.3) | 21 (17.4) | 0.23 | 0.88 |
| ŌĆāHepatocellular carcinoma | 4 (33.3) | 50 (38.5) | 1 (11.1) | 49 (40.5) | 0.08 | 0.73 |
| Comorbid condition | ||||||
| ŌĆāDiabetes mellitus | 1 (8.3) | 38 (29.2) | 3 (33.3) | 35 (28.9) | 0.78 | 0.12 |
| ŌĆāHypertension | 3 (25) | 14 (10.7) | 1 (11.1) | 13 (10.7) | 0.97 | 0.15 |
| Retransplantation | 0 | 4 (3.1) | 2 (22.2) | 2 (1.7) | < 0.01 | 0.53 |
| Pretransplant condition | ||||||
| ŌĆāMELD score | 21 (12.7ŌĆō30.7) | 18 (10ŌĆō30) | 28 (18.5ŌĆō39.5) | 16 (10ŌĆō28) | 0.05 | 0.56 |
| ŌĆāChild-Pugh, class C | 9 (75.0) | 77 (59.2) | 7 (77.9) | 70 (57.9) | 0.23 | 0.54 |
| ŌĆāPretransplant dialysis | 2 (16.7) | 23 (17.7) | 5 (55.6) | 18 (14.9) | < 0.01 | 0.92 |
| Posttransplant condition | ||||||
| ŌĆāReoperation | 1 (8.3) | 17 (13.1) | 3 (33.3) | 14 (11.6) | 0.06 | 0.64 |
| ŌĆāPosttransplant dialysis | 4 (36.4) | 18 (14.9) | 1 (12.5) | 17 (15.0) | 0.85 | 0.06 |
| ŌĆāBiliary complication | 5 (41.7) | 46 (35.4) | 2 (22.2) | 44 (36.4) | 0.39 | 0.66 |
| Invasive device | ||||||
| ŌĆāCentral venous catheter | 2 (16.7) | 40 (30.8) | 2 (22.2) | 38 (31.4) | 0.56 | 0.67 |
| ŌĆāHemodialysis catheter | 4 (33.3) | 23 (17.7) | 4 (44.4) | 19 (15.7) | 0.03 | 0.18 |
| ŌĆāFoley catheter | 6 (50.0) | 41 (31.5) | 2 (22.2) | 39 (32.2) | 0.53 | 0.19 |
| ŌĆāVentilator | 1 (8.3) | 3 (2.3) | 0 | 3 (2.5) | 0.64 | 0.23 |
| History of previous hospitalization | 3 (25.0) | 66 (50.8) | 6 (66.7) | 60 (49.6) | 0.32 | 0.08 |
| Preoperative hospital stay, day | 18 (4.5ŌĆō26) | 4 (3ŌĆō13) | 8 (1.3ŌĆō10.3) | 4 (3ŌĆō13) | 0.62 | 0.02 |
| ICU stay, day | 7 (4.3ŌĆō8) | 7 (5ŌĆō7) | 7 (5.3ŌĆō9.5) | 7 (5ŌĆō7) | 0.42 | 0.67 |
| Admission route | 0.01 | 0.85 | ||||
| ŌĆāICU | 0 | 3 (2.3) | 1 (11.1) | 2 (1.7) | ||
| ŌĆāEmergency department | 6 (50.0) | 60 (46.2) | 7 (77.8) | 53 (43.8) | ||
| ŌĆāGeneral ward | 6 (50.0) | 67 (51.5) | 1 (11.1) | 66 (54.5) | ||
Values are presented as median (interquartile range) or number (%). Percentages in parentheses represent the percentage of the total patient population, except for age and MELD score, which represent the range observed in the population.
MRSA, methicillin-resistant Staphylococcus aureus; MELD, Model for End-Stage Liver Disease; ICU, intensive care unit.
Table┬Ā3.
Comparison of clinical outcomes of patients who were initially colonized with MRSA (group 1a), acquired MRSA posttransplantation (group 2a), and were not colonized with MRSA (group 3a)
Table┬Ā4.
Risk factors involved in methicillin-resistant Staphylococcus aureus acquisition post-liver transplantation
Table┬Ā5.
Comparison of patients who were initially colonized by VRE (group 1b), acquired VRE posttransplantation (group 2b), and were not colonized with VRE (group 3b)
| Variable |
Initially colonize |
Not initially colonized |
p valuea | |||
|---|---|---|---|---|---|---|
| Group 1b (n = 37) | Total (n = 105) | Group 2b (n = 21) | Group 3b (n = 84) | p valueb | ||
| Age, yr | 50 (43.5ŌĆō56.5) | 49 (40.3ŌĆō56) | 51 (39.5ŌĆō55) | 49 (41ŌĆō56) | 0.91 | 0.42 |
| Male sex | 29 (78.4) | 64 (61.0) | 13 (61.9) | 51 (60.7) | 0.92 | 0.06 |
| Underlying liver disease | 0.30 | 0.33 | ||||
| ŌĆāHepatitis B | 23 (62.2) | 52 (49.5) | 9 (42.9) | 43 (51.2) | ||
| ŌĆāHepatitis C | 2 (5.4) | 9 (8.6) | 2 (9.5) | 7 (8.3) | ||
| ŌĆāHepatitis B + C | 1 (2.7) | 0 | 0 | 0 | ||
| ŌĆāHepatitis A | 3 (8.1) | 13 (12.4) | 2 (9.5) | 11 (13.1) | ||
| ŌĆāCryptogenic cirrhosis | 1 (2.7) | 4 (3.8) | 1 (4.8) | 3 (3.6) | ||
| ŌĆāPrimary biliary cirrhosis | 1 (2.7) | 0 | 0 | 0 | ||
| ŌĆāAlcoholic liver disease | 4 (10.8) | 15 (14.3) | 3 (14.3) | 12 (14.3) | ||
| ŌĆāOthers | 2 (5.4) | 12 (11.4) | 4 (19.0) | 8 (9.5) | ||
| Cause of transplantation | ||||||
| ŌĆāDecompensated liver cirrhosis | 25(67.6) | 54 (51.4) | 9 (42.9) | 45 (53.6) | 0.38 | 0.08 |
| ŌĆāFulminant hepatitis | 4(10.8) | 22 (21.0) | 4 (19.0) | 18 (21.4) | 0.81 | 0.17 |
| ŌĆāHepatocellular carcinoma | 12(32.4) | 42 (40.0) | 8 (38.1) | 34 (40.5) | 0.84 | 0.41 |
| Comorbid condition | ||||||
| Diabetes mellitus | 13 (35.1) | 26 (24.8) | 7 (33.3) | 19 (22.6) | 0.31 | 0.22 |
| Hypertension | 3 (8.1) | 14 (13.3) | 4 (19.0) | 10 (11.9) | 0.38 | 0.40 |
| Retransplantation | 1 (2.9) | 3 (2.9) | 1 (4.8) | 2 (2.4) | 0.56 | 0.99 |
| Pretransplant condition | ||||||
| ŌĆāMELD score | 22 (16ŌĆō30.7) | 16 (10ŌĆō29) | 14 (9.5ŌĆō31) | 16 (10ŌĆō27) | 0.89 | 0.02 |
| ŌĆāChild-Pugh, class C | 23 (62.2) | 63 (60.0) | 11 (52.4) | 52 (61.9) | 0.67 | 0.81 |
| ŌĆāPretransplant dialysis | 7 (18.9) | 18 (17.1) | 5 (23.8) | 13 (15.5) | 0.37 | 0.81 |
| Posttransplant condition | ||||||
| ŌĆāReoperation | 5 (13.5) | 13 (12.4) | 4 (19.0) | 9 (10.7) | 0.30 | 0.86 |
| ŌĆāPosttransplant dialysis | 5 (13.5) | 17 (16.1) | 4 (23.5) | 13 (16.3) | 0.47 | 0.66 |
| ŌĆāBiliary complication | 16 (43.2) | 35 (33.3) | 6 (28.6) | 29 (34.5) | 0.61 | 0.28 |
| Invasive device | ||||||
| ŌĆāCentral venous catheter | 10 (27.0) | 33 (31.4) | 6 (28.6) | 27 (32.1) | 0.76 | 0.62 |
| ŌĆāHemodialysis catheter | 8 (21.6) | 19 (18.1) | 4 (19.0) | 15 (17.9) | 0.90 | 0.64 |
| ŌĆāFoley catheter | 14 (37.8) | 33 (31.4) | 4 (19.0) | 29 (34.5) | 0.17 | 0.48 |
| ŌĆāVentilator | 0 | 4 (3.8) | 1 (4.8) | 3 (3.6) | 0.80 | 0.23 |
| History of previous hospitalization | 19 (51.4) | 50 (47.6) | 11 (52.4) | 39 (46.4) | 0.63 | 0.70 |
| Preoperative hospital stay, day | 11 (4ŌĆō18) | 4 (2.8ŌĆō11) | 4.5 (4ŌĆō7.5) | 4 (2ŌĆō13) | 0.55 | < 0.01 |
| ICU stay, day | 6 (5ŌĆō7) | 7 (5ŌĆō7) | 7 (6ŌĆō9) | 4 (5ŌĆō7) | 0.05 | 0.57 |
| Admission route | 0.78 | 0.24 | ||||
| ŌĆāICU | 0 | 3 (2.9) | 1 (4.8) | 2 (2.4) | ||
| ŌĆāEmergency department | 21 (56.8) | 45 (42.9) | 8 (38.1) | 37 (44.0) | ||
| ŌĆāGeneral ward | 16 (43.2) | 5 (54.3) | 12 (57.1) | 45 (53.6) | ||
Values are presented as median (interquartile range) or number (%). Percentages in parentheses represent the percentage of the total patient population, except for age and MELD score, which represent the range observed in the population.
VRE, vancomycin-resistant enterococci; MELD, Model for End-Stage Liver Disease; ICU, intensive care unit.
Table┬Ā6.
Comparison of patients who were initially colonized with VRE (group 1b), acquired VRE posttransplantation (group 2b), and were not colonized with VRE (group 3b)
Table┬Ā7.
Risk factors involved in vancomycin-resistant enterococci acquisition posttransplantation
REFERENCES
1. Singh N, Paterson DL, Chang FY, et al. Methicillin-resistant Staphylococcus aureus: the other emerging resistant gram-positive coccus among liver transplant recipients. Clin Infect Dis 2000;30:322ŌĆō327.


2. Kim YJ, Kim SI, Wie SH, et al. Infectious complications in living-donor liver transplant recipients: a 9-year single-center experience. Transpl Infect Dis 2008;10:316ŌĆō324.


3. Haddadin AS, Fappiano SA, Lipsett PA. Methicillin resistant Staphylococcus aureus (MRSA) in the intensive care unit. Postgrad Med J 2002;78:385ŌĆō392.



4. Niven DJ, Laupland KB, Gregson DB, Church DL, S aureus Screening Initiative Group. Epidemiology of Staphylococcus aureus nasal colonization and influence on outcome in the critically ill. J Crit Care 2009;24:583ŌĆō589.


5. Kim YJ, Kim SI, Kim YR, Lee JY, Park YJ, Kang MW. Risk factors for vancomycin-resistant enterococci infection and mortality in colonized patients on intensive care unit admission. Am J Infect Control 2012;40:1018ŌĆō1019.


6. Weinstock DM, Conlon M, Iovino C, et al. Colonization, bloodstream infection, and mortality caused by vancomycin-resistant enterococcus early after allogeneic hematopoietic stem cell transplant. Biol Blood Marrow Transplant 2007;13:615ŌĆō621.


7. Chang FY, Singh N, Gayowski T, Drenning SD, Wagener MM, Marino IR. Staphylococcus aureus nasal colonization and association with infections in liver transplant recipients. Transplantation 1998;65:1169ŌĆō1172.


8. Desai D, Desai N, Nightingale P, Elliott T, Neuberger J. Carriage of methicillin-resistant Staphylococcus aureus is associated with an increased risk of infection after liver transplantation. Liver Transpl 2003;9:754ŌĆō759.


9. Russell DL, Flood A, Zaroda TE, et al. Outcomes of colonization with MRSA and VRE among liver transplant candidates and recipients. Am J Transplant 2008;8:1737ŌĆō1743.


10. Hashimoto M, Sugawara Y, Tamura S, et al. Acquisition of methicillin-resistant Staphylococcus aureus after living donor liver transplantation: a retrospective cohort study. BMC Infect Dis 2008;8:155.




11. Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control 1988;16:128ŌĆō140.


12. Willey BM, Jones RN, McGeer A, et al. Practical approach to the identification of clinically relevant Enterococcus species. Diagn Microbiol Infect Dis 1999;34:165ŌĆō171.


13. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Nineteenth Informational Supplement M100-S18. Wayne: Clinical and Laboratory Standards Institute, 2009.
14. Seo JY, Kim PW, Lee JH, et al. Evaluation of PCR-based screening for vancomycin-resistant enterococci compared with a chromogenic agar-based culture method. J Med Microbiol 2011;60(Pt 7):945ŌĆō949.


15. McNeil SA, Malani PN, Chenoweth CE, et al. Vancomycin-resistant enterococcal colonization and infection in liver transplant candidates and recipients: a prospective surveillance study. Clin Infect Dis 2006;42:195ŌĆō203.


16. Woeste G, Zapletal C, Wullstein C, Golling M, Bechstein WO. Influence of methicillin-resistant Staphylococcus aureus carrier status in liver transplant recipients. Transplant Proc 2005;37:1710ŌĆō1712.


17. Santoro-Lopes G, de Gouvea EF, Monteiro RC, et al. Colonization with methicillin-resistant Staphylococcus aureus after liver transplantation. Liver Transpl 2005;11:203ŌĆō209.


18. Polisena J, Chen S, Cimon K, McGill S, Forward K, Gardam M. Clinical effectiveness of rapid tests for methicillin resistant Staphylococcus aureus (MRSA) in hospitalized patients: a systematic review. BMC Infect Dis 2011;11:336.




19. Bert F, Bellier C, Lassel L, et al. Risk factors for Staphylococcus aureus infection in liver transplant recipients. Liver Transpl 2005;11:1093ŌĆō1099.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



