 |
 |
| Korean J Intern Med > Volume 30(6); 2015 > Article |
|
See editorial "Time to learn from the past and prepare for the future in Helicobacter pylori eradication" on page 789.
Abstract
Background/Aims:
Trends in successful eradication of Helicobacter pylori using first-line triple therapy, consisting of a proton pump inhibitor, amoxicillin, and clarithromycin, have been understudied. We evaluated H. pylori eradication rates at a single center over the last 10 years and identified risk factors related to eradication failure.
Methods:
This study included 1,413 patients who were diagnosed with H. pylori infection and received 7 days of triple therapy between January 2003 and December 2012. We investigated H. pylori eradication rates retrospectively with respect to the year of therapy, as well as demographic and clinical factors. H. pylori eradication was confirmed by a 13C-urea breath test or a rapid urease test at least 4 weeks after the completion of triple therapy.
Results
The overall H. pylori eradication rate was 84.9%. Annual eradication rates from 2003 to 2012 were 93.5%, 80.0%, 87.2%, 88.5%, 92.0%, 88.3%, 85.7%, 84.1%, 83.7%, and 78.8%, respectively, by per-protocol analysis. The eradication rate with first-line triple therapy decreased during the last 10 years (p = 0.015). Multivariate analysis showed that female gender (odds ratio [OR], 1.69; 95% confidence interval [CI], 1.12 to 2.55) and smoking (OR, 1.61; 95% CI, 1.05 to 2.47) were associated with the failure of H. pylori eradication therapy.
Helicobacter pylori is a unique pathogen that lives in the acidic environment of the gastric mucosa. Infection with H. pylori can cause not only chronic gastritis, peptic ulcers, and mucosa-associated lymphoid tissue (MALT) lymphoma but also gastric cancer [1]. Thus, H. pylori has been classified as a definite gastric carcinogen (group I) by the International Agency for Research on Cancer, a branch of the World Health Organization [2,3]. Eradication of H. pylori decreases the risk for gastritis, peptic ulcers, and gastric cancers. Thus, H. pylori eradication is important to promote public health, especially in areas with high H. pylori prevalence.
The efficacy of therapy should be more than 80%, at least for first-line H. pylori eradication therapy, and proton pump inhibitor (PPI)-based triple therapy is one of the recommended regimens for first-line eradication therapy [4]. Specifically, PPI-based triple therapy, usually consisting of a PPI, amoxicillin, and clarithromycin, is a widely recommended regimen for H. pylori treatment in areas where clarithromycin resistance is low [5]. Although antibiotic resistance rates increased for amoxicillin (6.3% to 14.9%) and clarithromycin (17.2% to 23.7%) from 2003 through 2012 [6], eradication rates using clarithromycin-containing triple therapy were reported as 77.0% to 85.5% in Korea [7-11]. Under these circumstances, triple therapy consisting of a PPI, amoxicillin, and clarithromycin continues to be the recommended first-line treatment for H. pylori in Korea [12].
Several widely accepted studies have reported that poor compliance and antibiotic resistance are connected with eradication failure [13,14]. However, the relationship between eradication failure and other factors, such as gender, age, alcohol, smoking, and specific drug history (e.g., aspirin), are still controversial [10,14,15], and needing further research.
We investigated trends in H. pylori eradication rates in a single center over the last 10 years and determined risk factors related to the failure of eradication therapy.
Participants who visited Kosin University Gospel Hospital from January 2003 to December 2012, were diagnosed with a H. pylori infection, and received first-line PPI-containing triple therapy were enrolled retrospectively. This study protocol was approved by the Institutional Review Board of Kosin University Gospel Hospital.
H. pylori positivity was confirmed by a rapid urease test (CLO test, Delta West, Bentley, Australia) or a 13C-urea breath test before and after eradication therapy. We preformed the rapid urease test using a gastric mucosal biopsy from antrum and/or corpus. The endoscopic biopsy site was normal or near-normal gastric mucosa with less atrophy and intestinal metaplasia. Compliance was divided into good and poor by pill count based on medical records. Participants who took ≥ 80% of the prescribed medicine were deemed to belong in the good compliance group and those who took < 80% of the prescribed medicine were considered to be poorly compliant. Participants were excluded if they were poorly compliant or lost to follow-up. They were also excluded if they had received 14 days of first-line PPI-containing triple therapy or another antibiotic-based eradication therapy, such as 1 g amoxicillin, 500 mg clarithromycin, and 250 mg metronidazole twice daily.
We also evaluated demographic information, alcohol and smoking habits, comorbidities (e.g., diabetes mellitus or hypertension), area of residence, endoscopic diagnoses, and side effects of eradication therapy. Urban residence was determined as living in the metropolitan cities of Korea, and rural residence was presumed if participants did not live in the metropolitan cities. Endoscopic findings such as gastric ulcers, duodenal ulcers, gastric and duodenal ulcers, previous endoscopic submucosal dissection (ESD) state due to adenoma or early gastric cancer (EGC), MALT lymphoma, gastritis, dyspepsia, and gastric polyps were identified by gastroscopy or gastroscopy with biopsy. Side effects after eradication therapy were documented by investigating medical records.
Participants with H. pylori infection were recommended for eradication therapy consisting of a standard-dose PPI, 1 g amoxicillin, and 0.5 g clarithromycin twice daily for 7 days. Then, a rapid urease test or a 13C-urea breath test was performed to evaluate H. pylori eradication at least 4 weeks after the completion of the treatment.
To verify H. pylori infection with the rapid urease test (CLO test) an endoscopic biopsy was conducted on the gastric mucosa. The tissue sample was immersed in the rapid urea reagent. If the color of the reagent changed from yellow to red at least 12 hours later, the result was positive. If there was no change in the color, the result was negative.
Before the examination, participants fasted for at least 4 hours before the first breath sample was collected. Then, participants took tablets containing 100 mg of 13C-urea (UBiT, Otsuka Pharmaceutical, Tokyo, Japan) with 100 mL water orally. At 20 minutes after taking the tablets, the second breath sample was obtained. H. pylori infection was analyzed by 13C-urea breath test (UBiT-IR300, Otsuka Electronics, Osaka, Japan) using the collected breath samples. The cut-off value of the procedure was 2.5‰.
All statistical analyses were performed with the SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). The H. pylori eradication rate was demonstrated on a per-protocol analysis, and the trend in H. pylori eradication rates were analyzed with linear-by-linear association. Categorical variables were analyzed by using a chi-square test and continuous variables were analyzed using Student t test. Univariate and multivariate logistic regression were used for the analysis of risk factors, which were expressed as the odds ratio (OR) and 95% confidence intervals (CI). The p values ≤ 0.05 were considered to indicate statistical significance.
Between January 2003 and December 2012, 1,413 participants were diagnosed with H. pylori infection and received 7 days of first-line triple therapy. The average age (mean ± standard deviation) was 54.5 ± 12.1 years (range, 14 to 86), and 512 patients (36.2%) were female. Many patients had endoscopic diagnoses, including gastric ulcers (488, 34.5%), duodenal ulcers (426, 30.1%), gastric and duodenal ulcers (127, 9.0%), and previous ESD state due to adenoma or EGC (218, 15.4%). Other conditions included MALT lymphoma, gastritis, dyspepsia, and gastric polyps (154, 10.9%). The clinical data and demographic features are shown in Table 1.
Among 1,413 participants receiving first-line triple therapy, in total, 1,199 participants achieved successful eradication using a per-protocol analysis. The overall H. pylori eradication rate was 84.9% with first-line triple therapy. The annual eradication rates from 2003 to 2012 were 93.5%, 80.0%, 87.2%, 88.5%, 92.0%, 88.3%, 85.7%, 84.1%, 83.7%, and 78.8%, respectively, by a per-protocol analysis. The eradication rate in relation to first-line triple therapy decreased over the years (p = 0.015). Fig. 1 presents the annual eradication rates for the last 10 years.
In terms of endoscopic diagnoses, there was no statistically significant difference in eradication rates versus endoscopic diagnoses, such as gastric ulcers, duodenal ulcers, gastric and duodenal ulcers, previous ESD state due to adenoma or EGD, and others (MALT lymphoma, gastritis, dyspepsia, and gastric polyps; p = 0.297) (Table 2).
Of the 1,413 participants, 55 participants (3.9%) complained of adverse events after eradication therapy. Adverse events possibly associated with the treatment were diarrhea in 22 patients, bloating or abdominal pain in 18 patients, nausea or vomiting in five patients, and others (such as myalgia, headache, and bitter sensation in the mouth) in 10 patients (Table 3).
Factors possibly related to eradication failure are summarized in Table 4. In univariate analysis, there was no statistically significant clinical factor associated with eradication failure. However, multivariate analysis revealed that female gender (OR, 1.72; 95% CI, 1.14 to 2.60; p = 0.010) and cigarette smoking (OR, 1.64; 95% CI, 1.07 to 2.52; p = 0.023) were significantly associated with eradication failure. In the multivariate analysis, we found no statistical difference in eradication failure rates related to age, residence, alcohol, diabetes mellitus, or hypertension.
This study revealed that the eradication rate using PPI-based triple therapy, consisting of a PPI, amoxicillin, and clarithromycin, has decreased over the last 10 years (p = 0.015). The overall H. pylori eradication rate was 84.9%, but the eradication rate was 93.5% in 2003 and 78.8% in 2012. These efficacies were similar to the results of other Korean studies. Some studies have shown a statistically significant decline in annual eradication rate [7,16] while others showed no statistical difference [8,9]. Several factors, such as age, gender, and socioeconomic factors, have been proposed to affect H. pylori eradication rates. The most important correlative factor is antibiotic resistance [17]. A report from Japan investigated the relationship between eradication rates and primary resistance to clarithromycin from 1997 to 2008, divided into four terms. This study found that eradication rates declined significantly, from 90.6% to 80.2%, 76.0%, and 75.8% over the four periods, while clarithromycin resistance rates increased significantly, from 8.7% to 23.5%, 26.7%, and 34.5% [18]. In Korea, primary resistance rates have increased to amoxicillin (6.3% to 14.9%) and clarithromycin (17.2% to 23.7%) from 2003 through 2012 [6]. Rates of H. pylori eradication were 67.9% for clarithromycin-resistant strains and 95.5% for the clarithromycin-sensitive strains in another Korean study [19]. Thus, antibiotic resistance, especially clarithromycin resistance, is a major factor in determining the efficacy of H. pylori eradication with PPI-based triple therapy. In Korea, clarithromycin resistance varied depending on the geographic region. Busan, where our institute is located, had the highest rate of clarithromycin resistance (42.1%) [20]. We did not evaluate antibiotic resistance of H. pylori in the current study, but it may have influenced our results. Acceptable first-line H. pylori eradication therapy should have a success rate of at least 80%. If the success rate decreases to less than 80%, the region is recommended to empirically avoid this treatment regimen [4]. The overall H. pylori eradication rate was 84.9% in our study; the eradication rate with clarithromycin-containing triple therapy in Korea overall has been reported as 85.5% [7], 84.5% [9], 81.6% [11], 78.7% [8], and 77.0% [10]. Recent guidelines for the treatment of H. pylori infection in Korea consider PPI-based triple therapy for 7 to 14 days as first-line H. pylori eradication therapy [21]. However, the efficacy of PPI-based triple therapy for H. pylori infection has decreased in recent years; thus, other first-line therapies might be required for H. pylori eradication in the near future.
Our study showed that female gender (OR, 1.72; 95% CI, 1.14 to 2.60) and smoking (OR, 1.64; 95% CI, 1.07 to 2.52) were associated with eradication failure. Some studies revealed that women have lower H. pylori eradication rates when receiving a PPI, metronidazole, and amoxicillin triple therapy [22,23]. In a Korean study, the eradication rate of H. pylori-infected patients with the A2143G mutation in the 23S rRNA receiving clarithromycin-containing triple therapy was zero [19]. Another study also reported an 87.5% eradication failure rate in patients with the A2143G mutation who were treated with clarithromycin. Additionally, women were preferentially infected with H. pylori carrying the A2143G mutation in this study (p < 0.005) [24]. Thus, this point mutation in the 23S rRNA in H. pylori strains infecting women can contribute to the failure of PPI based triple therapy including clarithromycin.
Smoking has been proposed as a risk factor for H. pylori eradication failure [14,15,25]. It is hypothesized that decreased gastric blood flow and secretion of mucus related to smoking could reduce the delivery of antibiotics to the gastric mucosa, thereby decreasing the efficacy of eradication therapy [14,26]. Furthermore, amoxicillin, which was prescribed in our study, is an acid-sensitive antibiotic; as smoking provokes acid secretion, the effect of amoxicillin may be decreased, resulting in eradication failure [15,27]. Independently, smoking is a recognized determinant of eradication failure related to poor compliance [13,15], which is, itself, an important factor in eradication failure [14].
Limitations of the current study are we did not diagnose H. pylori by histology before and after eradication therapy, and only a few patients underwent both the rapid urease test and the 13C-urea breath test for confirmation of H. pylori. These limitations could affect the eradication rate. However, either the rapid urease test or the 13C-urea breath test were used to confirm H. pylori presence before and after therapy. The reported sensitivity and specificity of the rapid urease test were 90% to 95% and 95% to 100%, respectively, producing only rare false-positive tests [28-30]. In addition, the accuracy of both tests are high and very convenient for clinical use [31]. Thus, the absence of histology might not have had any significant effect on the study.
In conclusion, the efficacy of first-line triple therapy, consisting of a PPI, amoxicillin, and clarithromycin, for H. pylori infection has decreased over the last 10 years. We conclude that the rates of antibiotic resistance in H. pylori have increased, particularly with respect to clarithromycin. Additionally, females and smokers are at higher risk for eradication failure with PPI-based triple therapy. Although further studies on a larger scale are needed, other first-line therapies may need to be considered for H. pylori eradication in the near future in Korea.
1. This study showed the Helicobacter pylori eradication rate has decreased over the last 10 years.
2. The failure of H. pylori eradication therapy can be influenced by female gender and smoking.
3. This is the first study on this matter conducted in the southeast of the Korea, in areas such as Gyeongsangnam-do and Busan.
Acknowledgments
We thank our participants and the Institutional Review Board in our hospital for participation in, and consideration of, the present study. We also thank the Alumni Association of Kosin Medical College, which has continued to encourage our study team.
Figure 1.
Eradication rates of Helicobacter pylori with first-line triple therapy with time. The trend in eradication rates with first-line triple therapy decreased over the years (p = 0.015).
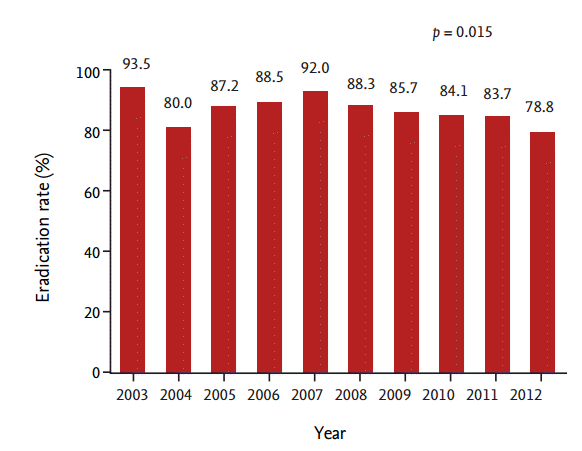
Table 1.
Baseline characteristics of participants (n = 1,413a)
| Characteristic | Value |
|---|---|
| Age, yr | 54.5 ± 12.1 |
| Sex | |
| Male | 901 (63.8) |
| Female | 512 (36.2) |
| Cigarette smoking | 422/1,121 (37.6) |
| Alcohol intake | 513/1,121 (45.8) |
| Diabetes mellitus | 102/1,130 (9.0) |
| Hypertension | 219/1,130 (19.4) |
| Residence | |
| Rural | 282 (20.0) |
| Urban | 1,131 (80.0) |
| Endoscopic diagnosis | |
| Gastric ulcer | 488 (34.5) |
| Duodenal ulcer | 426 (30.1) |
| Gastric ulcer + duodenal ulcer | 127 (9.0) |
| Post ESD due to adenoma or EGC | 218 (15.4) |
| Othersb | 154 (10.9) |
Table 2.
Eradication rates according to the endoscopic diagnosis (n = 1,413)
| Endoscopic diagnosis | Value |
|---|---|
| Gastric ulcer | 424/488 (86.9) |
| Duodenal ulcer | 359/426 (84.3) |
| Gastric ulcer + duodenal ulcer | 103/127 (81.1) |
| Post ESD due to adenoma or EGC | 179/218 (82.1) |
| Othersa | 134/154 (87.0) |
Table 3.
Side effects after Helicobacter pylori eradication therapy (n = 1,413)
| Side effect | No. (%) |
|---|---|
| Diarrhea | 22 (1.6) |
| Bloating or abdominal pain | 18 (1.3) |
| Nausea or vomiting | 5 (0.4) |
| Othersa | 10 (0.7) |
| Total | 55 (3.9) |
Table 4.
Clinical factors related with eradication failure of proton pump inhibitor-based triple therapy
| Variable | Eradication success (n = 1,199) | Eradication failure (n = 214) | Univariate p value | Multivariate p value | Adjusted OR (95% CI)a |
|---|---|---|---|---|---|
| Age, yr | 0.873 | 0.393 | 1.18 (0.81–1.72) | ||
| < 50 | 372 (85.1) | 65 (14.9) | |||
| ≥ 50 | 827 (84.7) | 149 (15.3) | |||
| Sex | 0.089 | 0.010b | 1.72 (1.14–2.60)b | ||
| Male | 776 (86.1) | 125 (13.9) | |||
| Female | 423 (82.6) | 89 (17.4) | |||
| Residence | 0.853 | 0.362 | 0.83 (0.56–1.24) | ||
| Rural | 238 (84.4) | 44 (15.6) | |||
| Urban | 961 (85.0) | 170 (15.0) | |||
| Cigarette smoking | 0.171 | 0.023b | 1.64 (1.07–2.52)b | ||
| No | 600 (85.8) | 99 (14.2) | |||
| Yes | 349 (82.7) | 73 (17.3) | |||
| Alcohol intake | 0.740 | 0.587 | 1.12 (0.74–1.69) | ||
| No | 517 (85.0) | 91 (15.0) | |||
| Yes | 432 (84.2) | 81 (15.8) | |||
| Diabetes mellitus | 0.475 | 0.333 | 1.33 (0.75–2.38) | ||
| No | 872 (84.8) | 156 (15.2) | |||
| Yes | 84 (82.4) | 18 (17.6) | |||
| Hypertension | 0.168 | 0.448 | 0.72 (0.45–1.15) | ||
| No | 766 (84.1) | 145 (15.9) | |||
| Yes | 189 (86.3) | 30 (13.7) |
REFERENCES
1. Moss SF, Malfertheiner P. Helicobacter and gastric malignancies. Helicobacter 2007;12 Suppl 1:23–30.

2. IARC working group on the evaluation of carcinogenic risks to humans: some industrial chemicals. Lyon, 15-22 February 1994. IARC Monogr Eval Carcinog Risks Hum 1994;60:1–560.


3. Kim MS, Kim N, Kim SE, et al. Long-term follow-up Helicobacter pylori reinfection rate and its associated factors in Korea. Helicobacter 2013;18:135–142.


4. Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut 2010;59:1143–1153.


5. Prasertpetmanee S, Mahachai V, Vilaichone RK. Improved efficacy of proton pump inhibitor-amoxicillin-clarithromycin triple therapy for Helicobacter pylori eradication in low clarithromycin resistance areas or for tailored therapy. Helicobacter 2013;18:270–273.


6. Lee JW, Kim N, Kim JM, et al. Prevalence of primary and secondary antimicrobial resistance of Helicobacter pylori in Korea from 2003 through 2012. Helicobacter 2013;18:206–214.


7. Song JG, Lee SW, Park JY, et al. Trend in the eradication rates of Helicobacter pylori infection in the last 11 years. Korean J Med 2009;76:303–310.
8. Choi YS, Cheon JH, Lee JY, et al. The trend of eradication rates of first-line triple therapy for Helicobacter pylori infection: single center experience for recent eight years. Korean J Gastroenterol 2006;48:156–161.

9. Na HS, Hong SJ, Yoon HJ, et al. Eradication rate of first-line and second-line therapy for Helicobacter pylori infection, and reinfection rate after successful eradication. Korean J Gastroenterol 2007;50:170–175.

10. Cho DK, Park SY, Kee WJ, et al. The trend of eradication rate of Helicobacter pylori infection and clinical factors that affect the eradication of first-line therapy. Korean J Gastroenterol 2010;55:368–375.


11. Cho HJ, Bae RC, Lee SH, et al. The trend in the eradication rates of first- and second-line therapy for Helicobacter pylori infection in Daegu and Kyungpook provinces: a single center experience for the most recent 9 years. Korean J Med 2009;76:186–192.
12. Kim N, Kim JJ, Choe YH, et al. Diagnosis and treatment guidelines for Helicobacter pylori infection in Korea. Korean J Gastroenterol 2009;54:269–278.


13. Graham DY, Lew GM, Malaty HM, et al. Factors influencing the eradication of Helicobacter pylori with triple therapy. Gastroenterology 1992;102:493–496.


14. Moayyedi P, Chalmers DM, Axon AT. Patient factors that predict failure of omeprazole, clarithromycin, and tinidazole to eradicate Helicobacter pylori. J Gastroenterol 1997;32:24–27.


15. Suzuki T, Matsuo K, Ito H, et al. Smoking increases the treatment failure for Helicobacter pylori eradication. Am J Med 2006;119:217–224.


16. Yoon JH, Baik GH, Sohn KM, et al. Trends in the eradication rates of Helicobacter pylori infection for eleven years. World J Gastroenterol 2012;18:6628–6634.



17. Perri F, Villani MR, Festa V, Quitadamo M, Andriulli A. Predictors of failure of Helicobacter pylori eradication with the standard 'Maastricht triple therapy'. Aliment Pharmacol Ther 2001;15:1023–1029.


18. Sasaki M, Ogasawara N, Utsumi K, et al. Changes in 12-year first-line eradication rate of helicobacter pylori based on triple therapy with proton pump inhibitor, amoxicillin and clarithromycin. J Clin Biochem Nutr 2010;47:53–58.



19. Hwang TJ, Kim N, Kim HB, et al. Change in antibiotic resistance of Helicobacter pylori strains and the effect of A2143G point mutation of 23S rRNA on the eradication of H. pylori in a single center of Korea. J Clin Gastroenterol 2010;44:536–543.


20. Kim JY, Kim NY, Kim SJ, et al. Regional difference of antibiotic resistance of helicobacter pylori strains in Korea. Korean J Gastroenterol 2011;57:221–229.


21. Kim SG, Jung HK, Lee HL, et al. Guidelines for the diagnosis and treatment of Helicobacter pylori infection in Korea, 2013 revised edition. Korean J Gastroenterol 2013;62:3–26.


22. Osato MS, Reddy R, Reddy SG, Penland RL, Malaty HM, Graham DY. Pattern of primary resistance of Helicobacter pylori to metronidazole or clarithromycin in the United States. Arch Intern Med 2001;161:1217–1220.


23. Cai W, Zhou L, Ren W, Deng L, Yu M. Variables influencing outcome of Helicobacter pylori eradication therapy in South China. Helicobacter 2009;14:91–96.

24. Kim T, Song HJ, Shin SY, et al. Clarithromycin-resistant Helicobacter pylori associated with 23S rRNA point mutations in Jeju Island. Korean J Gastroenterol 2013;61:252–258.


25. Broutet N, Tchamgoue S, Pereira E, Lamouliatte H, Salamon R, Megraud F. Risk factors for failure of Helicobacter pylori therapy: results of an individual data analysis of 2751 patients. Aliment Pharmacol Ther 2003;17:99–109.


26. Holm L, Perry MA. Role of blood flow in gastric acid secretion. Am J Physiol 1988;254(3 Pt 1):G281–G293.


27. Labenz J, Stolte M, Blum AL, et al. Intragastric acidity as a predictor of the success of Helicobacter pylori eradication: a study in peptic ulcer patients with omeprazole and amoxicillin. Gut 1995;37:39–43.



28. Versalovic J. Helicobacter pylori. Pathology and diagnostic strategies. Am J Clin Pathol 2003;119:403–412.


29. Gatta L, Vakil N, Ricci C, et al. Effect of proton pump inhibitors and antacid therapy on 13C urea breath tests and stool test for Helicobacter pylori infection. Am J Gastroenterol 2004;99:823–829.


-
METRICS

- Related articles
-
Can Helicobacter pylori eradication affect long-term mortality?2021 May;36(3)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


