 |
 |
| Korean J Intern Med > Volume 30(6); 2015 > Article |
|
To the Editor,
Pulmonary sclerosing hemangioma is a rare neoplasm of the lungs first described in 1956 [1]. Recent immunohistochemical studies have revealed that these tumors are epithelial in origin and derived from primitive respiratory epithelium, incompletely differentiated type II pneumocytes, or Clara cells [2]. Although composed of mixed cell populations, clonal analysis has shown that these tumors are monoclonal [3]. However, there have been no reports of extrapulmonary metastases from these tumors to date. Herein, we describe a patient with bone metastasis from a pulmonary sclerosing hemangioma.
In January 2003, a 73-year-old female presented with abnormal chest X-ray findings. Two years prior, she had been diagnosed at another institution with a solitary pulmonary nodule, but refused further evaluation. She was a non-smoker and otherwise asymptomatic. Physical examination revealed normal results and no cervical, supraclavicular, axillary, or inguinal adenopathy. Computed tomography (CT) of the chest confirmed the presence of a solitary well-defined round mass in the superior segment of the right lower lobe with subcarinal lymph node enlargement (Fig. 1). The mass exhibited focal calcifications and substantial enhancement. Following percutaneous needle biopsy, the patient was diagnosed with pulmonary sclerosing hemangioma. Although surgical resection was recommended, the patient refused surgery and was lost to follow-up.
In February 2006, she revisited the hospital with progressed disease. A CT scan showed a lung mass that had increased in size with interlobar and subcarinal lymph node involvement. She underwent tumor resection consisting of a right, middle and lower lobectomy with lymph node dissection. The resected tumor was ovoid and well-circumscribed with a tongue-like invasion into the surrounding lung tissue. The cut surface was solid, inhomogeneous and myxoid, with yellowish tan, red and blue areas. Microscopic features were typical of pulmonary sclerosing hemangioma. The tumor was composed of oval to spindle-shaped stromal cells and surface cells resembling type II pneumocytes. One area of the tumor contained distinct foci of stromal cell overgrowth, forming cell balls mimicking a paraganglioma. Immunohistochemical staining was positive for epithelial membrane antigen, cytokeratin and thyroid transcription factor-1, and negative for CD34 and HMB45, findings that are consistent with previous reports of pulmonary sclerosing hemangioma. The Ki-67 labeling index was less than 5%. Pathologic examination revealed metastases in three of 18 peribronchial and mediastinal nodes. Histologic and immunohistochemical features of the nodal metastases were morphologically identical to those of the main mass.
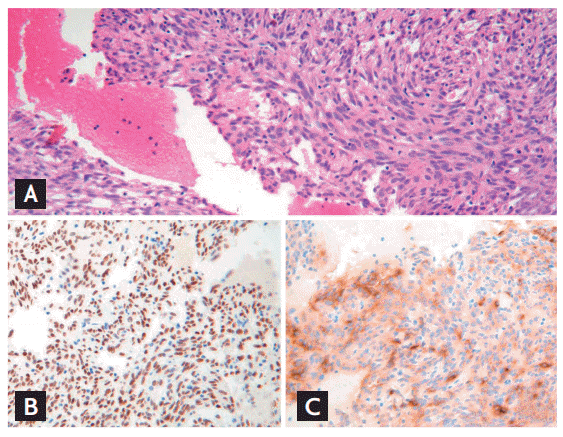
Six months later, the patient suffered a spontaneous compression fracture of the third lumbar vertebra. Spine magnetic resonance imaging (MRI) revealed a lesion in the right pedicle and lamina, with T1-low and T2-high signal intensity, and irregular enhancement in the L3 vertebral body (Fig. 2). Since the MRI could not rule out a pathologic compression fracture, we performed a whole body positron emission tomography (PET)/CT scan, which showed increased metabolic activity at L3 in the spine with a maximal standardized uptake value of 2.3. Histologic examination of a core needle biopsy specimen taken from the L3 vertebral body revealed a metastatic tumor composed of oval to spindle-shaped stromal cells morphologically identical to the stromal cells observed in the pulmonary mass (Fig. 3). Immunohistochemical staining also showed patterns identical to those of the stromal cells of the pulmonary sclerosing hemangioma. The patient underwent radiotherapy of the L2-S2 spine (180 cGy given 25 times, up to 4,500 cGy). The patient is currently undergoing follow-up examinations at our outpatient unit without evidence of recurrence.
Pulmonary sclerosing hemangioma occurs more frequently in Asians than in Western women. Its incidence peaks in the fifth decade of life, is often asymptomatic, and is frequently detected as a solitary well-circumscribed nodule on chest X-ray. CT is useful for further characterization of these tumors, which generally have varying areas of hyper-, iso-, and hypoattenuation, although marked contrast enhancement is typical. Several case reports have noted positive PET findings in patients with pulmonary sclerosing hemangioma [4]. The metastatic tumor in our patient also demonstrated substantial fluorodeoxyglucose uptake.
The treatment of choice for pulmonary sclerosing hemangioma is surgical resection; most hemangiomas follow a benign clinical course, with only 2% to 4% of patients having nodal metastases that do not appear to affect prognosis [5]. To date, there have been no reports of extrapulmonary metastases.
Microscopically, pulmonary sclerosing hemangiomas show varying histological profiles, which may include papillary, sclerotic, solid, and hemorrhagic patterns. These tumors are composed of two distinct cell populations: solid-growing polygonal cells and surface cuboidal cells that line the papillary structure. It has been reported that the two cell types may be derived from a common precursor cell, which undergoes divergent differentiation towards type II pneumocytes during tumorigenesis.
The histology of the pulmonary mass in our patient strongly resembled a sclerosing hemangioma with stromal cell overgrowth, and the histology of the spinal lesion was identical to a portion of the lung mass. Moreover, the immunohistochemical features of the spinal lesion were identical to those of the pulmonary tumor, indicating that the spinal tumor was a metastasis from the primary pulmonary sclerosing hemangioma. Although the mechanism by which this tumor acquired metastatic potential is not yet clear, our findings suggest two possibilities. First, the sclerosing hemangioma itself may have metastatic potential, as is seen in several other tumor types, such as pleomorphic adenomas of the salivary gland, which are regarded as benign tumors with metastatic potential. Second, part of the tumor may have transformed to a malignant phenotype and metastasized to distant organs, as seen in carcinoma ex pleomorphic adenoma. In our case, the overgrown stromal component within the pulmonary mass, which was accompanied by some aggressive features such as increased cellularity and necrotic areas, was likely to have metastasized to the spine. In contrast, the metastatic lymph nodes were histologically identical to the usual sclerosing hemangioma pattern within the pulmonary mass, and did not show any features typical of malignant neoplasms. Therefore, the clinicopathologic features of this tumor suggest that a type of spindle cell malignancy may have arisen from the stromal components within the pulmonary sclerosing hemangioma and metastasized to the bone. These findings indicate that a pulmonary sclerosing hemangioma can metastasize not only to lymph nodes with benign histologic features, but also to bone with a malignant histology. Further studies, including molecular analyses, should be performed in order to predict the specific biologic behavior of pulmonary sclerosing hemangiomas.
In conclusion, to our knowledge, this is the first report to describe the extrapulmonary metastatic potential of pulmonary sclerosing hemangiomas. Since the biological behaviors of these tumors remain unclear, physicians should be cautious regarding management and follow-up.
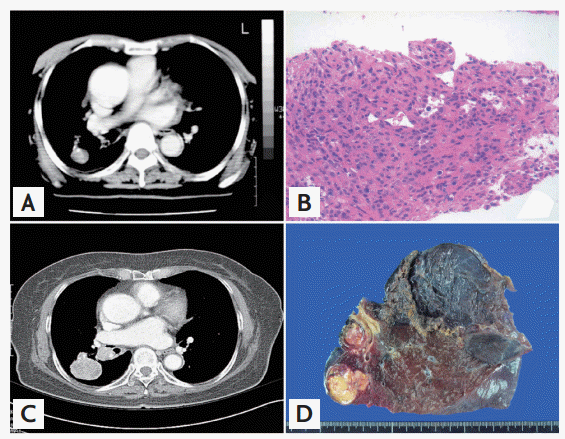
Figure┬Ā1.
Computed tomography scans and pathologic findings upon initial presentation in February 2001 and subsequently in February 2006. (A) A 3.5 cm well-enhanced soft tissue mass with focal calcification is present in the superior segment of the right lower lobe. (B) Needle biopsy of the tumor. Typical sclerosing hemangioma patterns composed of superficial cuboidal cells and round stromal cells are seen (H&E, ├Ś100). (C) A 4.0 cm mass with necrosis is present in the right lower lobe, together with enlargement of the lymph nodes in the right interlobar station. (D) Cross-section of the excised tumor. The resected tumor is yellowish tan in color and consists of myxoid tissue with multifocal hemorrhagic foci, common in sclerosing hemangiomas.
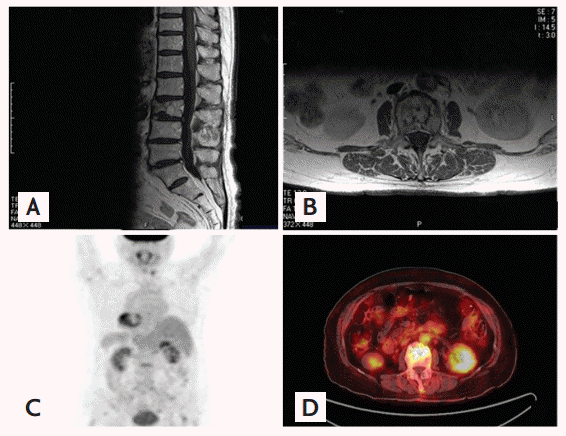
Figure┬Ā2.
Magnetic resonance imaging and positron emission tomography/computed tomography (PET/CT) scan of the lumbar spine. All images show a pathologic compression fracture in the third lumbar vertebra. (A) Sagittal section (T1WI) at the lumbosacral spine. (B) Axial section (T1WI) at the third lumbar vertebra. (C, D) PET/CT scan showing increased metabolic activity at the third lumbar vertebra.
REFERENCES
1. Liebow AA, Hubbell DS. Sclerosing hemangioma (histiocytoma, xanthoma) of the lung. Cancer 1956;9:53ŌĆō75.


2. Devouassoux-Shisheboran M, Hayashi T, Linnoila RI, Koss MN, Travis WD. A clinicopathologic study of 100 cases of pulmonary sclerosing hemangioma with immunohistochemical studies: TTF-1 is expressed in both round and surface cells, suggesting an origin from primitive respiratory epithelium. Am J Surg Pathol 2000;24:906ŌĆō916.


3. Niho S, Suzuki K, Yokose T, Kodama T, Nishiwaki Y, Esumi H. Monoclonality of both pale cells and cuboidal cells of sclerosing hemangioma of the lung. Am J Pathol 1998;152:1065ŌĆō1069.


-
METRICS

- Related articles
-
Acral Metastasis in a Patient with Ampullary Carcinoma2007 March;22(1)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


