 |
 |
| Korean J Intern Med > Volume 22(3); 2007 > Article |
|
Abstract
Background
Community-acquired pneumonia (CAP) remains a common and serious condition worldwide. The mortality from severe CAP remains high, and this has reached 50% in some series. This study was conducted to determine the mortality and predictors that contribute to in-hospital mortality for patients who exhibit CAP and acute respiratory failure that requires mechanical ventilation.
Methods
We retrospectively reviewed the medical records of 85 patients with severe CAP as a primary cause of acute respiratory failure, and this required mechanical ventilation in a setting of the medical intensive care unit (ICU) of a tertiary university hospital between 2000 and 2003.
Results
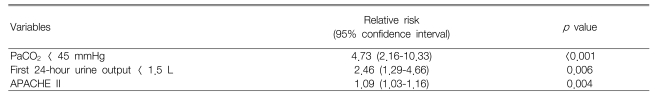
The overall in-hospital mortality was 56% (48/85). A Cox-proportional hazard model revealed that the independent predictive factors of in-hospital mortality included a PaCO2 of less than 45 mmHg (p<0.001, relative risk [RR]: 4.73; 95% confidence interval [CI]: 2.16-10.33), a first 24-hour urine output of less than 1.5 L (p=0.006, RR: 2.46, 95% CI: 1.29-4.66) and a high APACHE II score (p=0.004, RR: 1.09, 95% CI: 1.03-1.16).
Community-acquired pneumonia (CAP) is among the most frequent causes of hospitalization, and it is one of the most common causes of admission to intensive care units (ICUs)1, 2). Despite the advances in antibiotic therapy and supportive measures, the mortality rate for patients with severe pneumonia that requires admission to an ICU remains high, ranging from 29% to 55%3-7).
The most important risk factors for mortality include an advanced age, multilobar consolidation, respiratory failure, mechanical ventilation, shock, bacteremia, renal failure, an altered mental status, inadequate antimicrobial therapy and a high acute physiology and chronic health evaluation (APACHE) II score3-7). Many prognostic factors have been studied in different subpopulations, and the requirement for mechanical ventilation is known to be associated with increased mortality3-5). However, the prognostic factors for mortality have not been well investigated among the subgroup of patients who require mechanical ventilation8). Therefore, it is uncertain whether the prognostic criteria derived from mixed populations of ventilated and nonventilated patients are relevant for those specific patients who require mechanical ventilation. The rules that predict death from CAP, although far from perfect, should be adequate to identify the majority of patients with severe CAP and to provide useful support for the decision-making process. They may also contribute to the evaluation process and determining the outcomes of care for CAP patients, and they allow for better patient and patient family counseling9).
The objectives of this study were to determine the mortality and the relative importance of many factors that influence the survival of mechanically ventilated patients with severe CAP, and these factors include the baseline characteristics at admission and the organ failure that develops over the course of mechanical ventilation.
We retrospectively evaluated the consecutive patients with severe CAP and who required mechanical ventilation at admission or on the day following admission to the ICU at Ewha Womans University Hospital from January 1, 2000 through December 31, 2003. Those patients who had been pretreated before admission, those who had other causes of respiratory failure, those who were suspected of having nosocomial pneumonia or those who died within 24 hours of admission were excluded. Finally, 85 patients were included. The main outcome was hospital mortality. The clinical and laboratory characteristics at admission and during the course of mechanical ventilation were compared between the survivors and non-survivors. The selection of these variables was based on clinical experience and on a review of the published data3-8).
CAP was defined as an acute respiratory illness acquired in a non-hospital setting and it was manifested by characteristic symptoms (cough, sputum, dyspnea, pleuritic chest pain and/or an altered mental status) with the appearance of a new compatible radiographic infiltrate, accompanied by fever (>38.5℃) or hypothermia (<36.0℃), and/or an abnormally increased or decreased white blood cell (WBC) count10). Severe CAP was defined as pneumonia that required mechanical ventilation for one or more of the following reasons: hypoxemic respiratory failure (a PaO2 < 60 mmHg with the patient on maximal supplemental oxygen), hypercarbic respiratory failure (a pH < 7.25 with a PaCO2 > 50 mmHg) or an inability to protect the airway due to a depressed mental status. All the patients were investigated using an acute physiology and chronic health evaluation (APACHE) II questionnaire11).
Mean values and standard deviations were used to summarize the results of the continuous variables, and numbers and percentages were used to summarize the results for the categorical data. All the statistical results were produced using SPSS-PC (version 11.0, SPSS Inc, Chicago, IL). Independent two group comparisons were performed using the Student t-test, and the χ2 test was used to compare the categorical variables. We used Cox regression analysis for the survival analysis, and the relative risks and 95% confidence intervals (CI) were calculated. All the reported p values are two sided, and p values < 0.05 were considered statistically significant.
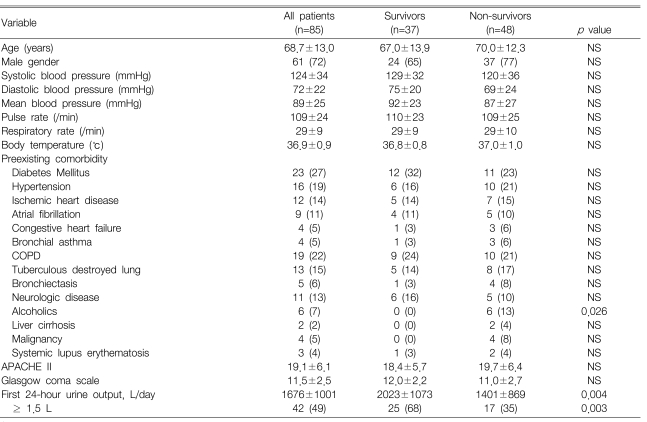
Among the 85 patients with pneumonia, 48 died for a mortality rate of 56%. At admission, no differences were observed between the survivors and non-survivors in terms of age, gender and the vital signs. However, alcoholics were less common among the survivors than the non-survivors (p=0.026). The survivors had a significantly larger first 24-hour urine output than did the non-survivors (p=0.004), and the survivors had a lower APACHE II score, though this was not statistically significant (Table 1).
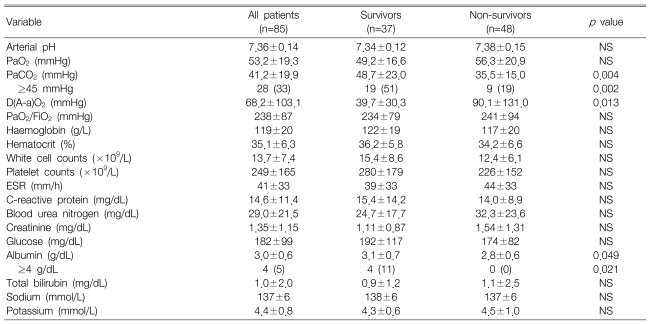
Arterial blood gas analyses showed that the survivors had a significantly higher PaCO2 (p=0.004) and lower alveolar-arterial oxygen differences [D(A-a)O2] than did the non-survivors (p=0.013), and the serum albumin level was higher in the survivors than that in the non-survivors (p=0.049) (Table 2).
The microbiologic results showed no difference between the survivors and non-survivors. Streptococcus pneumoniae was the most commonly isolated respiratory pathogen and Pseudomonas aeruginosa and methicillin-resistant Staphylococcus aureus were the next 2 most common pathogens.
No difference was evident between the survivors and non-survivors in terms of the time to start administering antibiotics. The initial antibiotic of choice was similar for the two groups, except for vacomycin/teicoplanin, which was more commonly given to the survivor group (p=0.02).
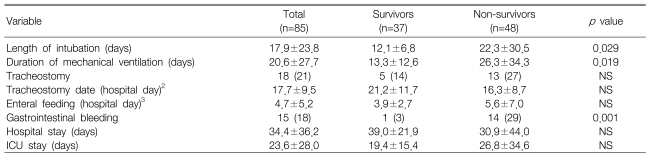
For the survivors, the number of days requiring endotracheal intubation (p=0.029) and mechanical ventilation were significantly less than that for the non-survivors (p=0.019). During hospitalization, a significantly lower incidence of gastrointestinal bleeding was noted for the survivors (p=0.001) (Table 3).
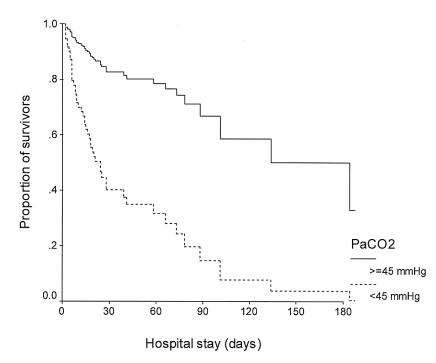
Cox regression survival analyses showed that a PaCO2 of less than 45 mmHg (p<0.001, relative risk [RR]=4.73, 95% confidence interval [CI]=2.16-10.33), a first 24-hour urine output of less than 1.5 L (p=0.006, RR=2.46, 95% CI=1.29-4.66) and a high APACHE II score (p=0.004, RR=1.09, 95% CI=1.03-1.16) were independently associated with death (Table 4, Figure 1, 2).
The mortality in our series that was attributable to severe CAP requiring mechanical ventilation was 56%. This rate was slightly higher than the overall mortality rates that have been quoted for similar populations, and these rates have ranged from 38% to 55%6, 8, 9, 12). This discrepancy may be explained by the high rate (88%) of comorbidities in our population; more that half of the patients had two concurrent illnesses or more. However, despite this possible overestimation, the mortality among patients with severe CAP, and in particular for those patients who required mechanical ventilation was undoubtedly high and this is a challenge for physicians in the ICU environment.
Cox regression analysis identified three prognostic factors, i.e., the PaCO2, the first 24hr urine output and the APACHE II score as being independently associated with mortality for the patients with severe CAP that required mechanical ventilation. Contrary to the previous investigations3-8, 13), it was interesting that age, the PaO2/FiO2 ratio, the blood urea and creatinine levels, the blood pressure, underlying disease, the serum albumin and the initial antibiotic therapy were not found to be predictive of mortality.
Of note, a normal to lower PaCO2 (<45 mmHg) was identified as the most important factor associated with higher mortality. This finding is at odds with the findings of Afessa et al14), who found that a high PaCO2 level was associated with higher mortality in patients with pneumococcal bacteremia. Moreover, another study recently demonstrated that in patients with CAP, both hypocapnia (PaCO2 <32 mmHg) and hypercapnea (PaCO2 ≥45 mmHg) increased mortality by twofold and threefold, respectively, compared with a normal PaCO2 (40 to 44 mmHg)15). These differences may be due to differences of the study populations. In our present study, all the patients received mechanical ventilation, whereas the previous studies also included non-ventilated patients. However, a few previous studies support our findings. Among the patients with tuberculous destroyed lung and who had been admitted to an ICU because of acute respiratory failure, a higher PaCO2 was found to be a predictor of improved survival16). Further, for patients who received long-term oxygen therapy due to pulmonary parenchymal disease, such as tuberculosis sequelae or interstitial fibrosis, a lower PaCO2 was found to indicate poorer survival17). Although almost 40% of our patients had a preexisting respiratory disease, and especially COPD or a tuberculous destroyed lung, no difference in the prevalence of these diseases was found between the survivors and non-survivors.
Ventilation is increased in patients with severe pneumonia, and this is primarily due to lung receptor stimulation, and probably also because of the lactic acidosis that arises from tissue hypoxia18). Despite the higher PaCO2 in the survivors of our study as compared to the non-survivors, the pH levels were similar in both groups. This implies that non-survivors with a relatively low PaCO2 might have a greater burden of metabolic acid than that of the survivors with a high PaCO2, which may have increased their respiratory drive18). Thus, hypocapnia and possibly even eucapnia may be markers of sepsis19). It is also possible that a lower PaCO2 at levels similar to hypoxemia indicates the presence of a more advanced diffusion disturbances or a more advanced ventilation-perfusion mismatch17). In the present study, the alveolar-arterial PO2 differences were significantly larger in the non-survivors than in the survivors. This data suggests that the ventilation-perfusion mismatch, which reflects deranged gas exchange, was more severe in the non-survivors. On the other hand, emerging evidence has indicated that hypercapnia in itself may be beneficial for patients with respiratory failure and inflammation20). Hypercapnia causes a complex interaction between altered cardiac output, hypoxic pulmonary vasoconstriction and intrapulmonary shunt, which causes a net increase in PaO221). Because hypercapnia increases the cardiac output, oxygen delivery is increased throughout the body21). Regional blood flow, including the mesenteric blood flow, is also increased22), and so this increases oxygen delivery to organs. In addition, because hypercapnia shifts the hemoglobin-oxygen dissociation curve to the right, oxygen delivery to tissues is further increased23). During inflammatory responses, hypercapnia may tilt the balance towards cell salvage at the tissue level24-26). Therefore, our results may indirectly reflect these beneficial effects of hypercapnia and particularly for patients with pneumonia complicated by respiratory failure.
A decreased first 24-hour urine output was the second important predictor of mortality, which is in agreement with the findings of El-solh et al6). Although a reduced urine out may reflect renal dysfunction, several plasma biochemical markers have been used to diagnose renal dysfunction, and plasma creatinine is known to be the most reliable marker of renal function. Thus, a large number of studies have shown that elevated blood urea and/or creatinine were highly correlated with a poor prognosis for patients with pneumonia3, 4, 11). However, in the present study, neither the blood urea nitrogen level nor the creatinine level differed between the survivors and non-survivors, and they were not correlated with the first 24-hour urine output. Mechanical ventilation with positive end-expiratory pressure (PEEP) is known to decrease the urine output and urinary sodium excretion because PEEP reduces atrial distension, which in turn reduces the release of atrial natriuretic factor27). Some investigators have consistently suggested that atrial natriuretic peptide improves pulmonary gas exchange by reducing the extravascular lung water28, 29). In other studies on patients receiving mechanical ventilation, a higher peak inspiratory pressure30) and a higher plateau airway pressure were associated with increased mortality9). In the present study, the non-survivors had slightly higher initial PEEP levels than did the survivors (the data is not shown). However, since we do not have exact pressure records such as auto-PEEP, or the peak and/or plateau airway pressures, we could not assume that the intrathoracic pressure, rather than the renal function per se could be related to urine output.
The third prognostic marker in our patients was an APACHE II score despite the lack of a difference between survivors and non-survivors. This finding concurs with those of others3, 11). However, contrary to the majority of studies on the subject5, 7, 8, 12), markers of oxygenation, i.e., PaO2, PaO2/FiO2 and an alveolar-arterial oxygen difference, were not found to be significantly associated with mortality in the present study. In addition, neither the specific pathogen type nor the initial antibiotic therapy was found to affect patient mortality. Moreover, the tracheostomy time and the time to starting enteral feeding were not found to have prognostic implications.
The present study has several limitations. First, it was a retrospective study that was conducted in the ICU of a university hospital, and some data such as airway pressure was not available. Second, the size of the study population was relatively small. Third, we did not have information on the specific causes of death. Fourth, as with most observational studies, there is a possibility of confounding by an unaccounted-for factor. However, our adjusted results were similar to our unadjusted results, which provide some assurance that significant confounding was unlikely.
In the present study and in most other studies on the subject, severe CAP requiring mechanical ventilation was found to have high mortality. We conclude that high arterial CO2 and a large first 24-hour urine output favor survival, whereas a high APACHE II score predicts mortality. Further clinical studies with prospective and randomized designs should be undertaken to confirm these results. Moreover, laboratory investigations may provide deeper insight into the significance of the PaCO2 level and the urine output for pneumonia associated with respiratory failure.
References
1. Ewig S, Torres A. Severe community-acquired pneumonia. Curr Opin Crit Care 2002. 8:453–460PMID : 12357115.


2. Restrepo MI, Jorgensen JH, Mortensen EM, Anzueto A. Severe community-acquired pneumonia: current outcomes, epidemiology, etiology, and therapy. Curr Opin Infect Dis 2001. 14:703–709PMID : 11964888.


3. Dahmash NS, Chowdhury MN. Re-evaluation of pneumonia requiring admission to an intensive care unit: a prospective study. Thorax 1994. 49:71–76PMID : 8153944.



4. Leroy O, Santre C, Beuscart C, Georges H, Guery B, Jacquier JM, Beaucaire G. A five-year study of severe community-acquired pneumonia with emphasis on prognosis in patients admitted to an intensive care unit. Intensive Care Med 1995. 21:24–31PMID : 7560469.


5. Roson B, Carratala J, Dorca J, Casanova A, Manresa F, Gudiol F. Etiology, reasons for hospitalization, risk classes, and outcomes of community-acquired pneumonia in patients hospitalized on the basis of conventional admission criteria. Clin Infect Dis 2001. 33:158–165PMID : 11418874.


6. El-Solh AA, Sikka P, Ramadan F, Davies J. Etiology of severe pneumonia in the very elderly. Am J Respir Crit Care Med 2001. 163:645–651PMID : 11254518.


7. Luna CM, Famiglietti A, Absi R, Videla AJ, Nogueira FJ, Fuenzalida AD, Gene RJ. Community-acquired pneumonia: etiology, epidemiology, and outcome at a teaching hospital in Argentina. Chest 2000. 118:1344–1354PMID : 11083685.


8. Pascual FE, Matthay MA, Bacchetti P, Wachter RM. Assessment of prognosis in patients with community-acquired pneumonia who require mechanical ventilation. Chest 2000. 117:503–512PMID : 10669697.


9. Esteban A, Anzueto A, Frutos F, Alia I, Brochard L, Stewart TE, Benito S, Epstein SK, Apezteguia C, Nightingale P, Arroliga AC, Tobin MJ. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28-day international study. JAMA 2002. 287:345–355PMID : 11790214.


10. Fine MJ, Orloff JJ, Arisumi D, Fang GD, Arena VC, Hanusa BH, Yu VL, Singer DE, Kapoor WN. Prognosis of patients hospitalized with community-acquired pneumonia. Am J Med 1990. 88:1N–8NPMID : 2294759.


11. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985. 13:818–829PMID : 3928249.


12. Laterre PF, Garber G, Levy H, Wunderink R, Kinasewitz GT, Sollet JP, Maki DG, Bates B, Yan SC, Dhainaut JF. Severe community-acquired pneumonia as a cause of severe sepsis: data from the PROWESS study. Crit Care Med 2005. 33:952–961PMID : 15891319.


13. Yoshimoto A, Nakamura H, Fujimura M, Nakao S. Severe community-acquired pneumonia in an intensive care unit: risk factors for mortality. Intern Med 2005. 44:710–716PMID : 16093592.


14. Afessa B, Greaves WL, Frederick WR. Pneumococcal bacteremia in adults: a 14-year experience in an inner-city university hospital. Clin Infect Dis 1995. 21:345–351PMID : 8562743.


15. Sin DD, Man SF, Marrie TJ. Arterial carbon dioxide tension on admission as a marker of in-hospital mortality in community-acquired pneumonia. Am J Med 2005. 118:145–150PMID : 15694899.


16. Park JH, Na JO, Kim EK, Lim CM, Shim TS, Lee SD, Kim WS, Kim DS, Kim WD, Koh Y. The prognosis of respiratory failure in patients with tuberculous destroyed lung. Int J Tuberc Lung Dis 2001. 5:963–967PMID : 11605892.

17. Strom K, Boman G. Long-term oxygen therapy in parenchymal lung diseases: an analysis of survival. Eur Respir J 1993. 6:1264–1270PMID : 8287941.


18. Caruana-Montaldo B, Gleeson K, Zwillich CW. The control of breathing in clinical practice. Chest 2000. 117:205–225PMID : 10631221.


19. Blair E. Hypocapnia and gram-negative bacteremic shock. Am J Surg 1970. 119:433–439PMID : 5437850.


20. Laffey JG, Kavanagh BP. Carbon dioxide and the critically ill:-too little of a good thing? Lancet 1999. 354:1283–1286PMID : 10520649.


21. Hickling KG, Joyce C. Permissive hypercapnia in ARDS and its effect on tissue oxygenation. Acta Anaesthesiol Scand Suppl 1995. 107:201–208PMID : 8599278.


22. Cardenas VJ Jr, Zwischenberger JB, Tao W, Nguyen PD, Schroeder T, Traber LD, Traber DL, Bidani A. Correction of blood pH attenuates changes in hemodynamics and organ blood flow during permissive hypercapnia. Crit Care Med 1996. 24:827–834PMID : 8706461.


23. Torbati D, Mangino MJ, Garcia E, Estrada M, Totapally BR, Wolfsdorf J. Acute hypercapnia increases the oxygen-carrying capacity of the blood in ventilated dogs. Crit Care Med 1998. 26:1863–1867PMID : 9824080.


24. Yamaguchi K, Takasugi T, Fujita H, Mori M, Oyamada Y, Suzuki K, Miyata A, Aoki T, Suzuki Y. Endothelial modulation of pH-dependent pressor response in isolated perfused rabbit lungs. Am J Physiol 1996. 270:H252–H258PMID : 8769759.


25. Parfenova H, Leffler CW. Effects of hypercapnia on prostanoid and cAMP production by cerebral microvascular cell cultures. Am J Physiol 1996. 270:C1503–C1510PMID : 8967453.


26. Rehncrona S, Hauge HN, Siesjo BK. Enhancement of iron-catalyzed free radical formation by acidosis in brain homogenates: differences in effect by lactic acid and CO2. J Cereb Blood Flow Metab 1989. 9:65–70PMID : 2492027.


27. Teba L, Dedhia HV, Schiebel FG, Blehschmidt NG, Lindner WJ. Positive-pressure ventilation with positive end-expiratory pressure and atrial natriuretic peptide release. Crit Care Med 1990. 18:831–835PMID : 2143130.


28. Mitaka C, Hirata Y, Habuka K, Narumi Y, Yokoyama K, Makita K, Imai T. Atrial natriuretic peptide improves pulmonary gas exchange by reducing extravascular lung water in canine model with oleic acid-induced pulmonary edema. Crit Care Med 2002. 30:1570–1575PMID : 12130981.


Figure 2
Survival curve during the hospital stay for different levels of urine output during the first 24-hours.
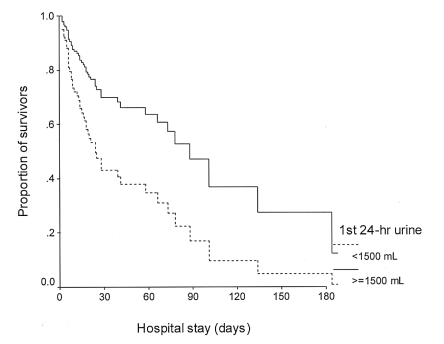
Table 2
Laboratory characteristics of the survivors and non-survivors with severe pneumonia at admission1



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



