 |
 |
| Korean J Intern Med > Volume 31(4); 2016 > Article |
|
To the Editor,
Ganglioneuroma (GN) of the gastrointestinal (GI) tract is rare tumor composed of ganglion cells, nerve fibers, and supporting cells of the enteric nervous system. Diffuse ganglioneuromatosis is associated with multiple endocrine neoplasia IIB (MEN IIB), neurofibromatosis type I (NF1, also known as von Recklinghausen disease), multiple cutaneous or GI tract neurofibromatosis, and neurogenic sarcoma of the GI tract [1]. However, usual presentation of GN is small mucosal polyp < 1 to 2 cm, or sometimes multiple polyps. Few cases of solitary polypoid GN were reported in the literature, and colonic mucosal GN associated with colonic polyps is a very unusual finding. We report here a case of polypoid GN associated with hyperplastic polyposis and discuss the important pathological features and clinical issues.
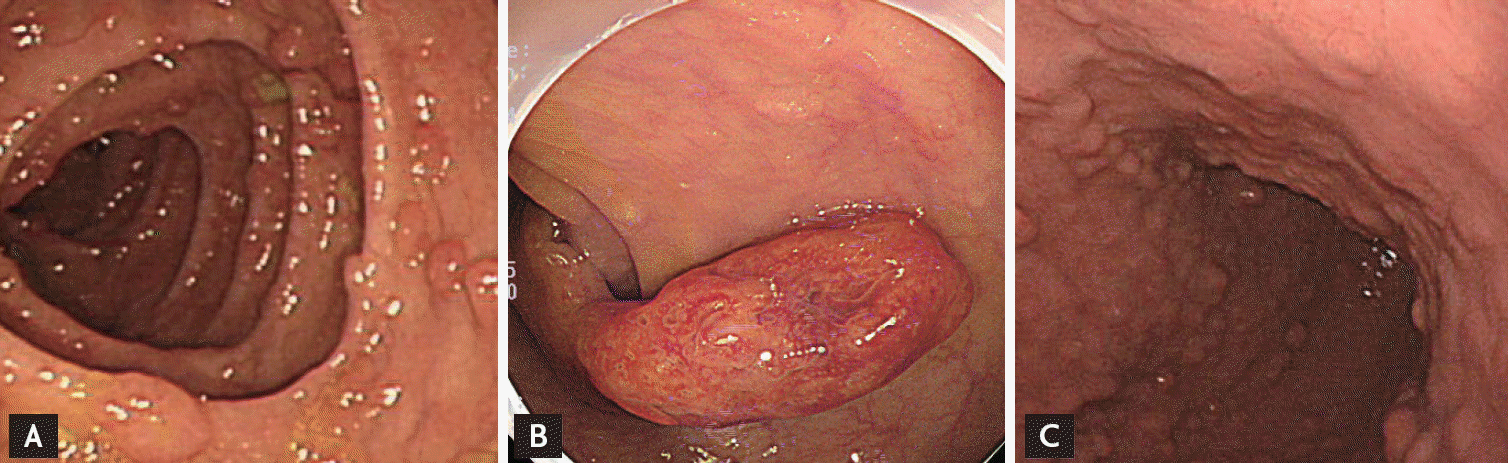
A 51-year-old Korean woman underwent colonoscopy for health checkup. The patient denied abdominal pain, diarrhea, or weight loss. She and her family had no history of familial adenomatous polyposis, MEN IIB, NF1, juvenile polyposis, or Cowden syndrome. During colonoscopy, numerous small sessile polyps were observed in the transverse colon, sigmoid colon, and rectum (Fig. 1A). The polyps were mainly located in rectosigmoid area. The colonoscpic biopsy was performed in polyps in size from 2 to 5 mm. Pathologic examination of 3 polyps in cecum, 1 polyp in ascending colon, 1 polyp in hepatic fracture, 2 polyps in sigmoid colon revealed hyperplastic polyps. Pedunculated polyp in sigmoid colon showed irregular lobulation with redness, measuring 3.0 ├Ś 1.5 cm in greatest dimensions (Fig. 1B). The endoscopic finding suggested differential diagnosis for hamartomatous polyp. The polypectomy was done after injection of normal saline and indigo carmine solution into the submucosal layer. The polyp was removed clearly by endoscopic mucosal resection. Microscopically, at low magnification the polyp showed disturbed crypt architecture with cystic glands, expanded lamina propria, and a smooth surface epithelium. Nerve ganglion and stromal cells were also noted in the lamina propira (Fig. 2A). Under closer inspection at higher magnification, collection of spindle cells in fibrillar matrix and irregular groups and nest of ganglion cells were observed specifically within the lamina propria (Fig. 2B). Immunohistochemically, the ganglion cells were positive for neuron specific enolase (NSE) (Fig. 2C) and S-100 protein (Fig. 2D). As a result of the colonoscopy, the patient also underwent esophagogastroduodenoscopy. Inner cavity of the stomach was covered with numerous sessile polyps were identified and biopsied (Fig. 1C). Pathologic examination of multiple gastric biopsies revealed hyperplastic polyps.
GN may be found anywhere in the body, particularly in the distribution of the major sympathetic ganglia. Involvement of the GI tract is a rare occurrence, although cases have been reported throughout the GI tract, from lip to the rectum. Most GI GN occurs in the colon with a predilection to the left side and the rectum, and a few are found in the appendix. Polypoid GN is mostly asymptomatic and observed during endoscopy or surgery. However, symptoms including rectal bleeding, abdominal pain, weight loss, bowel habit change could be presented according to tumor location and mass.
Shekitka and Sobin [1] reviewed 43 patients with GNs registered at the armed forced institute of pathology over 50 year period and classified them into three major categories: diffuse ganglioneuromatosis, ganglioneuromatous polyposis, and polypoid GN. Diffuse ganglioneuromatosis is poorly demarcated nodular and diffuse intramural to transmural proliferation of ganglioneuromatous tissue elements that involves the enteric plexuses [1]. These lesions are large (1 to 17 cm), poorly demarcated, and can distort the surrounding tissue architecture. Diffuse ganglioneuromatosis can exist as an isolated finding or related to MEN IIB [2] or NF1 [3]. Ganglioneuromatous polyposis refers to GN greater than 20 sessile or pedunculated mucosal and/or submucosal lesions [1]. Polypoid GN is small, sessile, or pedunculated polyps, grossly resembling hyperplastic polyps, juvenile polyps, or adenomas. Polypoid GN is solitary or few in numbers. Microscopically, these lesions show essential three patterns, including juvenile polyp like pattern, a neurofibromatous pattern, or a combination of both.
The diagnosis of GN is based on the identification of ganglion cells. Hematoxylin and eosin stain are usually sufficient for identifying the ganglion cells. However, NSE is helpful in the cases when ganglion cells are sparse and S-100 protein is useful in confirming the neural background and the extent of the lesion.
Polypoid GN with multiple adenomatous polyposis has been reported [4], but colonic mucosal GN associated with colonic polyps is a very unusual finding. Our patient has polypoid GN with hyperplastic polyposis that has not been reported. Although the possibility remains that hyperplastic polyps coexist with adenoma, biopsied polyps all revealed hyperplastic polyps except GN. However, relationship of the ganglion cells to the polyps in adjacent mucosa remains unclear regarding the case of polypoid GN with multiple adenomatous polyposis. Interestingly, reviewing pathologic findings of GNs with colonic polyps in the previous reports [1,4], GNs associated with multiple colon polyps including ours showed juvenile polyp like pattern microscopically regardless of the associated colon polyps. It is conceivable that presence of heterotopic ganglion cells in the lamina propria seemed to have been an underlying developmental anomaly which eventuated in polyp formation. This finding can be correspond to the explanation suggested by Shekitka and Sobin [1] which proposed hamartoma/choristoma theory for the solitary polypoid GN, because of the lack of ganglion cells in normal intestinal mucosa and prevalence of the polypoid GN to occur as mucosal based lesions.
Due to benign clinical behavior, complete resection is the treatment of choice for the polypoid GN, we planned next screening/surveillance endoscopy for the hyperplastic polyposis.
Figure┬Ā1.
(A) The colonoscpic finding revealed numerous small sessile polyps. (B) Polypoid ganglioneuroma presented as pedunculated polyp, measuring 3.0 ├Ś 1.5 cm. (C) Esophagogastroduodenoscopic findings revealed numerous polyps on the antrum of the stomach.
Figure┬Ā2.
Juvenile polyp associated with focal ganglioneuromatosis. (A) Hyperplastic and cystic glands typical of a juvenile polyp were shown. Expansion of the lamina propria by proliferating spindle cells is indicated by arrow (H&E, 1:1). (B) Higher-magnification revealed numerous ganglion cells set in a spindle stoma in the mucosa (H&E, ├Ś200). (C) The ganglion cells were positively stained with neuron specific enolase (├Ś200). (D) The ganglion cells also showed immunoreactivity for S-100 protein (├Ś100).

REFERENCES
1. Shekitka KM, Sobin LH. Ganglioneuromas of the gastrointestinal tract. Relation to Von Recklinghausen disease and other multiple tumor syndromes. Am J Surg Pathol 1994;18:250ŌĆō257.


2. Carney JA, Go VL, Sizemore GW, Hayles AB. Alimentary-tract ganglioneuromatosis: a major component of the syndrome of multiple endocrine neoplasia, type 2b. N Engl J Med 1976;295:1287ŌĆō1291.


-
METRICS

- Related articles
-
Splenic infarction associated with acute infectious mononucleosis2018 March;33(2)
Abnormal ectodermal findings associated with gastrointestinal polyposis2016 September;31(5)
Gastric polyposis associated with portal hypertension2013 March;28(2)
A Case of Hereditary Angioedema Associated with Idiopathic Hypoparathyroidism2001 December;16(4)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


