To the Editor,
BrunnerŌĆÖs gland hamartoma is a benign, polypoid proliferation of BrunnerŌĆÖs glands. Usually asymptomatic and discovered incidentally, these lesions may manifest occasionally as a cause of duodenal obstruction or upper gastrointestinal hemorrhage, which require surgical excision [1].
Furthermore, in spite of low propensity for malignant transformation [2], BrunnerŌĆÖs gland hamartoma can be confused with other malignancies, including duodenal adenocarcinoma or pancreatic head cancer [3].
BrunnerŌĆÖs gland hamartoma can be categorized into three types based on gross appearance, as follows: polypoid type, mass-forming type, and circumferential infiltrative type. Most clinical studies and case reports have examined the polypoid or mass-forming types of BrunnerŌĆÖs gland hamartoma, while few studies have focused on the circumferential infiltrative type [4]. In this case report, we present an unusual case of circumferential type BrunnerŌĆÖs gland hamartoma causing annular stricture of the duodenum, with symptoms of gastric outlet obstruction.
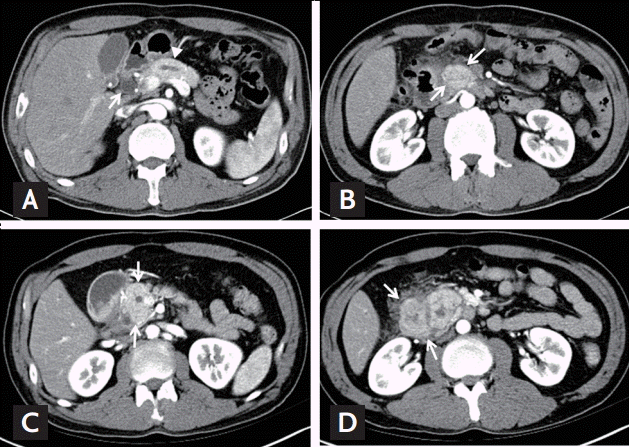
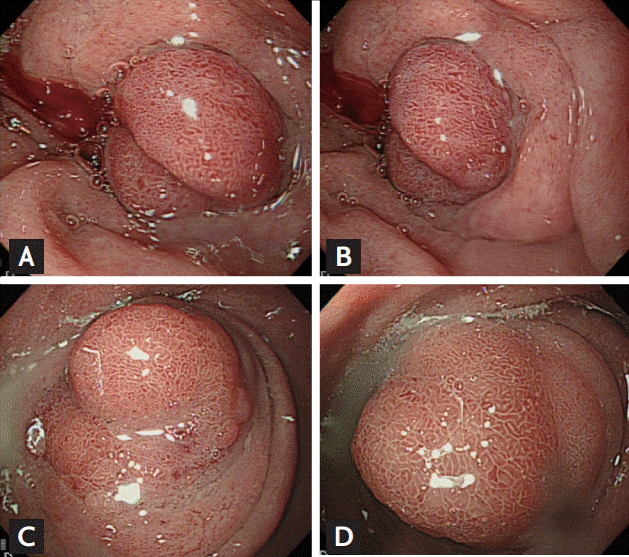
A 44-year-old man presented at our institution with recurrent vomiting and epigastric pain. He had been treated for similar symptoms 2 years prior; however, he had not fully recovered and was experiencing repeated episodes. He had no significant past medial history, but was an active smoker with a 30 pack years smoking history, and was a heavy alcoholic. When he was admitted to our hospital for the same symptoms 2 years prior, his laboratory values were within normal ranges, apart from elevated levels of gamma glutamyltranspeptidase, to 75 IU/L. His levels of amylase and lipase were within normal ranges. Moreover, abdominal computed tomography (CT) scans showed dilatation of the common bile duct and the main pancreatic duct (Fig. 1A) due to stenosis at the pancreas head portion and mild wall thickening of the duodenum proximal portion (Fig. 1B). Esophagogastroduodenoscopy (EGD) showed circumferential submucosal edema with the appearance of a protruding mass in the superior descending angle and second portion of the duodenum, which resulted in partial duodenal obstruction (Fig. 2A and 2B). Non-specific moderate duodenitis was identified in the histologic examination of the protruding mass. Given that the patient had a past medical history of acute pancreatitis and this episode occurred following heavy drinking, it seemed that chronic pancreatitis with acute exacerbation had led to partial duodenal obstruction. Conservative management was performed and his symptoms improved, and he was discharged without significant complications.
During this episode, the patient complained of further aggravation of his symptoms. Laboratory tests showed no significantly abnormal values, apart from mild elevations of alanine aminotransferase levels to 39 IU/L, amylase to 110 IU/L, and lipase to 101 IU/L. Cancer antigen 19-9 levels were within the normal range, while carcinoembryonic antigen levels were elevated to 9.19 ng/mL. The CT scans performed after admission showed dilatation of the common bile duct and the main pancreatic duct due to enlargement and stenosis at the pancreas head (Fig. 1C), as well as edema of the duodenal wall (Fig. 1D).
The lesion was not observed in the head of the pancreas. EGD revealed a polypoid mass with circumferential mucosal swelling with nearly complete obstruction of the duodenum (Fig. 2C and 2D). The size of the mass was similar to that found 2 years prior. Although severe groove pancreatitis was primarily suspected, the possibility of malignant transformation could not be completely excluded, as the symptoms of nausea and vomiting were severe and chronic. Considering that palliative surgery would be required for diagnostic and therapeutic purposes, we decided to transfer the patient to the Department of Surgery for treatment using a surgical approach. Pancreaticoduodenectomy was performed.
Circumferential enlargement of the duodenal mucosa was found in the proximal duodenum, in which the lesion was suspected to manifest as a cause of duodenal obstruction (Fig. 3A). Microscopically, the lesion was covered with small intestinal mucosa and composed of lobular proliferation of BrunnerŌĆÖs glands that were increased in both size and number, and were separated by thin fibrous septa. Some of the ducts were dilated and infiltration of lymphocytes with some lymphoid follicles was also observed (Fig. 3B). There was no evidence of pancreatic heterotopia in the duodenal wall. The pancreas showed no abnormal pathologic findings. As a result, the patient was diagnosed with a gastric outlet obstruction at the level of the superior descending angle due to a BrunnerŌĆÖs gland hamartoma, which enclosed the duodenal bulb circumferentially. After surgery, the patient recovered well and was discharged without significant complications.
BrunnerŌĆÖs glands are alkaline-secreting glands located in the submucosal layer. Although they extend from the pylorus to the third portion of the duodenum, the majority of them are located in the first portion and their prevalence decreases in the second and third portions. Histologically, they are branched acinotubular glands covered with cuboidal cells in the ducts and cuboidal to columnar clear cells in the glands [3]. The main physiological function of these tubuloalveolar glands includes the secretion of pepsinogen, urogastrone, and alkaline-based mucus in response to the acidic gastric contents passed from the stomach, to protect the duodenal mucosa.
In the present case, a large BrunnerŌĆÖs gland proliferation over 1 cm in diameter was identified in the specimen, which was eventually diagnosed as BrunnerŌĆÖs gland hamartoma. A BrunnerŌĆÖs gland hamartoma is a rare benign tumor of the duodenum. Thus far, cases of BrunnerŌĆÖs gland hamartoma have been reported sparsely in the literature (i.e., less than 200 cases reported). The distribution of BrunnerŌĆÖs gland hamartoma follows that of BrunnerŌĆÖs glands; approximately 70% of BrunnerŌĆÖs gland hamartomas were found in the duodenal bulb, followed by 26% in the second portion of the duodenum, and 4% in the third portion [3]. No gender or race differences were observed in the incidence of BrunnerŌĆÖs gland hamartoma. It mostly presents during the fifth and sixth decades of life, and is known to be rare in the pediatric age group [3].
The pathogenesis of BrunnerŌĆÖs gland hamartoma or hyperplasia remains unknown, although chronic inflammation caused by excessive secretion of gastric acid may play an important role [5]. Furthermore, Helicobacter pylori infections, uremia, and pancreatitis are also potentially associated with increased incidence of BrunnerŌĆÖs gland hamartoma [3]. In the present case, chronic pancreatitis may have resulted from chronic alcohol abuse, leading to the development of the large-sized hamartoma.
Although the majority of BrunnerŌĆÖs gland hamartoma are asymptomatic and are diagnosed incidentally, several clinical manifestations can occur depending on the size, type, and location, which include epigastric pain, obstruction and gastrointestinal bleeding, jaundice, and pancreatitis [2]. In this case, gastric outlet obstruction-related symptoms including epigastric pain, recurrent vomiting, and poor oral intake were present. BrunnerŌĆÖs gland hamartoma can be categorized into the following three groups: polypoid type, the mass-forming type, and circumferential infiltrative type. Considering the gross appearance of each type, obstructive symptoms are usually caused by the large polypoid type or the mass-forming type, and are very rarely associated with the circumferential type [4]. On the contrary, in the present case, the circumferentially proliferated BrunnerŌĆÖs gland hamartoma caused annular stricture in the duodenum, eventually leading to nearly complete obstruction of the duodenum.
Although endoscopy and radiologic examinations are usually used for the diagnosis of BrunnerŌĆÖs gland hamartoma, its detection is somewhat difficult in some cases and there are still many pathologic conditions for differential diagnosis, including duplication cysts, leiomyoma, leiomyosarcoma, adenoma or adenocarcinoma, lymphoma, gastrointestinal stromal tumors, aberrant pancreatic tissue, and chronic pancreatitis [3]. Specifically, it is difficult to differentiate between the circumferential infiltrative type of BrunnerŌĆÖs gland hamartoma and pancreatitis or neoplastic lesions. Definite diagnosis is possible only by histopathological examination. In this case, EGD and abdominal CT scans were employed for diagnosis; however, it remained difficult to differentiate from groove pancreatitis, malignancy, and autoimmune pancreatitis preoperatively. The final diagnosis of BrunnerŌĆÖs gland hamartoma was confirmed by pathologic report.
Despite some debate, conservative treatment with proton pump inhibitors and antacids can be sufficient for the treatment of BrunnerŌĆÖs gland hamartoma when the patient is asymptomatic [4]. However, several studies have demonstrated that symptomatic BrunnerŌĆÖs gland hamartoma cases can be successfully treated using a surgical approach, with either endoscopic or surgical resection [1]. Furthermore, the possibility of malignant transformation is rare, but exists. Brookes et al. [2] reported a case of BrunnerŌĆÖs gland hamartoma with multiple foci of dysplasia, suggesting that the probability of malignant transformation cannot be excluded for determining the treatment method. In the present case, as the obstructive symptoms and inflammation of the pancreas head were observed and the risk of malignancy could not be excluded, pancreaticoduodenectomy was performed and the patient recovered completely.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





