To the Editor,
As first-line therapy in patients with metastatic renal cell carcinoma (mRCC), particularly for patients with clear cell carcinoma, the National Comprehensive Cancer Network recommends one of sunitinib, temsirolimus, or bevacizumab in combination with interferon, pazopanib, high dose of interleukin 2 (IL-2), or sorafenib. If primary treatment fails, subsequent therapies might differ based on the type of agent used in first-line therapy. Everolimus is recommended as second-line treatment when tyrosine kinase inhibitor therapy is not sufficiently effective. The RECORD 1 trial is an international, multicenter, double-blind, randomized phase III trial, comparing everolimus and placebo for the treatment of metastatic RCC in patient who had failed treatment with sunitinib or sorafenib. In this study, the primary end point was progression-free survival (PFS), and everolimus showed better PFS, 4.0 months versus 1.9 months. Therefore, the demand for everolimus in kidney cancer treatment has increased tremendously [1].
Everolimus, an orally administered mammalian target of rapamycin (mTOR) inhibitor, acts as an immunosuppressive agent and proliferation signal inhibitor. It is also used for patients that received solid organ transplantation. Hepatitis B virus (HBV) reactivation has been reported in some patients with kidney transplant taking everolimus as an immunosuppressant. HBV reactivation is common among immunocompromised patients and steps for its prevention are well established. It is recommended that HBV carrier patients start taking antiviral agents anywhere between before chemotherapy to 6 month after the last chemotherapy to prevent HBV reactivation.
Recently, reactivation of latent tuberculosis infection (LTBI) was reported in patients who received a kidney transplant [2,3]. The typical everolimus dose in patients with organ transplant is 3 mg/day, whereas for patients with mRCC, it is 10 mg/day. Patients exposed to higher doses of everolimus are at a greater risk of being immunocompromised, allowing LTBI reactivation. Therefore, before prescribing everolimus to patients with mRCC, oncologists should be concerned about the prevention of LTBI reactivation. Here, we report a case of LTBI reactivation in a patient with mRCC taking everolimus and anti-tuberculosis (TB) medication.
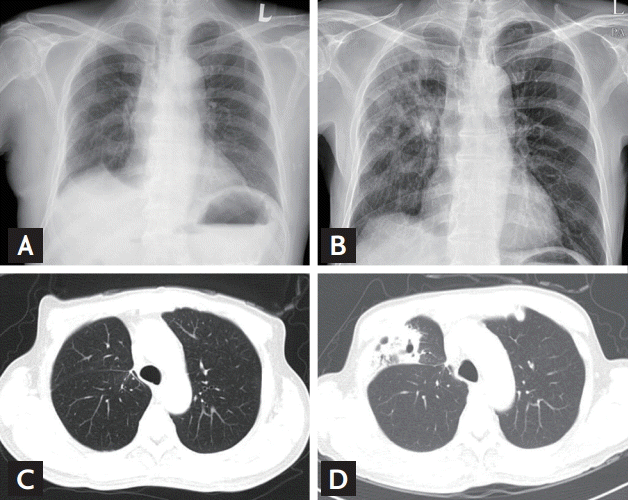
A 64-year-old man was admitted to our hospital with fever, cough, nasal drip, and sputum. Chest X-ray (CXR) exam showed pneumonia with cavitary lesions (Fig. 1). While reviewing his history, we noted that he was diagnosed with stage I kidney cancer, by abdomen computed tomography (CT), and underwent radical nephrectomy 5 years ago. The pathological report indicated a diagnosis of clear renal cell carcinoma (ccRCC). For 3 years, no recurrence of renal cell carcinoma was observed. However, 3 years after surgery, a surveillance CT indicated recurrence of the cancer with metastases to the lung and pancreas. He was surgically treated by metastasectomy, pylorus-preserving pancreaticoduodenectomy, and right upper lobectomy. The pathological report for both sites indicated metastatic ccRCC. Resected lung tissue also showed a granuloma, which was positive for TB-polymerase chain reaction (PCR) and acid-fast stain (AFB) stain. However, the patient presented no clinical symptoms and his bronchial washing fluid culture was negative for TB. There was no evidence of active TB. Thus, rather than prescribing anti-TB medication, the patient was placed under active surveillance.
After the second surgery, the patient was diagnosed with stage IV cancer with distant metastases, and was treated with sunitinib. Fourteen months later, newly developed multiple lung metastases and adrenal gland metastases were found by CT. Thus, sunitinib was replaced by everolimus, 10 mg/day. Seventy-five days after taking everolimus, he visited the hospital complaining of fever, cough, nasal drip, and sputum. CXR and high resolution CT (HRCT) showed a cavitary lesion in the lung right middle lobe (Fig. 1). The sputum and bronchial washing fluid were 3+ for AFB stain. The patient was diagnosed with active TB, identified as Mycobacterium tuberculosis complex. We started the anti-TB therapy with isoniazid, rifabutin, ethambutol, and pyrazinamide. Four weeks later, susceptibility test for antibiotics indicated resistance to isoniazid. Thus, the regimen was changed to rifabutin/ethambutol/pyrazinamide/moxifloxacin for 8 weeks, and changed again to ethambutol/pyrazinamide/moxifloxacin/cycloserin and maintained for 9 months.
By the 4th month of TB medication, lung metastasis progression was observed. Thus, we replaced everolimus by pazopanib, which was taken for 21 months. Again, the disease progressed. Consequently, we again changed the treatment from pazopanib to temsirolimus.
The World Health Organization announced that approximately one-third of the worldŌĆÖs population has latent TB. Although the incidence of TB is decreasing, the declining rate is very slow and TB infection is the second most common cause of death next to human immunodeficiency virus and acquired immune deficiency (HIV/AIDS) infection as single infectious diseases. TB is fatal and, for patients transplanted with solid organs, LTBI reactivation is a challenging problem [2]. LTBI reactivation incidence in patients with solid organ transplant has been reported in the literature. The authors advocate LTBI therapy for transplant candidates. Everolimus inhibits IL-2-driven T-cell proliferation by arresting cells in the G1 to S phase of the cell cycle. The effective and safe dose of everolimus for patients transplanted with solid organs is typically 3 mg/day. Everolimus also has cytostatic or cytotoxic effects on certain tumors and has been approved by the United State Food and Drug Administration for the treatment of mRCC, breast cancer, rhabdomyosarcoma, and primitive neuroectodermal tumor. The dose of everolimus as an anticancer agent is 10 mg/day. This dosage was established from a phase I pharmacokinetic and pharmacodynamic study of everolimus in solid organ malignancy. The dose of everolimus for patients with mRCC is approximately three times higher than the dose for patients with organ transplant. Thus, patients with mRCC should be more immunocompromised. Of course as mentioned above, LTBI reactivation is common problem for solid organ transplantation who taking immunosuppressant. The incidence of use of higher dose of everolimus in mRCC is increasing, the more attention is needed for this chronic TB infection.
The test for latent TB infection should be considered in some situations such as for individuals presenting with immunocompromised diseases like HIV, those in contact with patients with active TB, and those undergoing immunosuppressant treatment. Indeed, in several rheumatic disorders, physicians screen for latent TBI using tests such as CXR, TB skin test, also called purified-protein derivative skin test, and interferon gamma release assay test before the use of a tumor necrosis factor (TNF) antagonist (infliximab, etanercept, and adalimumab). When the LTBI test is positive, isoniazid prophylaxis should be started to prevent LTBI reactivation [4]. For systemic chemotherapy, there is no recommended guideline for the prophylaxis of TB reactivation. Thus, the treatment is generally started only after TB reactivation.
As mentioned earlier the dose of everolimus for patients with organ transplant is typically 3 mg/day, whereas it is 10 mg/day for patients with mRCC. The dose for patients with mRCC is approximately three times higher than that for patients with an organ transplant. Thus, patients with mRCC are more likely to be immunocompromised. As more cases of TB reactivation are reported, the need for preventive protocols should increase. Additionally, LTBI routine screening and prophylaxis should be considered if TNF antagonist is prescribed for treatment. Future studies should focus on the effectiveness of prophylaxis for LTBI reactivation and personalized therapy.
In endemic TB areas, the incidence of LTBI reactivation is much higher. Our patient experienced everolimus-induced LTBI reactivation. Before lung surgery, the patientŌĆÖs HRCT revealed old TB lesion, and the lesion removed by metastasectomy. After lobectomy, we could not observe the TB lesion in image study, but the possibility of invisible LTBI could not be ruled out. And the patient did not have close contact with any active TB patients, therefore, TB reactivation was more likely rather than TB reinfection. Currently, everolimus use is increasing not only for treatment of patients with mRCC, but also for patients with other cancers such as breast cancer and sarcomas. Some experts recommend investigating possible LTBI through patient medical history, Mantoux reaction, and QuantiFERON-TB Gold test (QFT-G, Cellestis Limited, Carnegie, Australia), particularly in endemic countries or for migrants originating from these countries [5]. The incidence of everolimus-induced LTBI reactivation is not clear. Thus, a large-scale study is necessary to evaluate the incidence of LTBI reactivation caused by everolimus. Moreover, the results of such study could provide decisive guidelines for screening LTBI reactivation and prophylaxis in patients undergoing everolimus treatment.
Rifampin can cause drug-drug interaction frequently. Rifampin and rifabutin, alternative to rifampin, are representative strong CYP3A4 inducer, and lower the blood levels of everolimus. There are very few data on concurrent use of rifampin and mTOR inhibitors. Everolimus is recommended to avoid concurrent use with rifampin or rifabutin, but if simultaneous use is essential, the recommended dose of everolimus is twice its usual dose, but closer therapeutic drug monitoring (TDM) of everolimus is required [3]. In this case, the patient taken rifabutin and everolimus concurrently, the dose of everolimus was maintained 10 mg/day, because TDM of everolimus was not available.
In conclusion, everolimus can induce LTBI reactivation, but its incidence remains unclear. Furthermore, more in-depth studies of everolimus-induced LTBI reactivation are warranted to provide screening and prophylaxis guidelines.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





