 |
 |
| Korean J Intern Med > Volume 31(4); 2016 > Article |
|
Abstract
Background/Aims:
Increasing incidences of hepatitis C virus (HCV) infection in human immunodeficiency virus (HIV)-infected men who have sex were reported in the United States and Europe. However, few studies regarding the epidemiology of HCV infection in HIV-infected patients in Asian countries have been reported.
Methods:
To determine the prevalence and incidence of HCV infection in HIV-infected patients, a retrospective cohort study was conducted. All HIV-infected patients who visited a tertiary care hospital in Korea from 2000 to 2013 were identified. Patients with Ōēź 1 HCV antibody (Ab) test were included and observed until December 2014.
Results:
Among 996 HIV-infected patients, 790 patients (79%) had baseline HCV Ab tests and 41 (5.2%) were positive at baseline and four at follow-up. Experience of injecting drug use (IDU; adjusted odds ratio, 16.20; 95% confidence interval [CI], 1.56 to 167.89; p < 0.01) was significantly associated with prevalent HCV infection. Conversion to HCV Ab positivity was observed in four of 384 included patients, with an incidence rate of 2.22 (95% CI, 0.60 to 5.80)/1,000 person-years (PYs); 164.89 (95% CI, 34.00 to 481.88)/1,000 PYs in patients with IDU, and 1.40 (95% CI, 0.35 to 7.79)/1,000 PYs in men who have sex with men who denied IDU. There was no significant increase in incidence rate of HCV in HIV-infected patients from 2009 to 2014 (p = 0.119). Among 19 patients who were positive for HCV RNA, genotype 1b (73%) was the most common following 2a/2c (20%).
Coinfection with human immunodeficiency virus (HIV) and hepatitis C virus (HCV) is common since both infections share similar routes of transmission. Patients with HCV and HIV coinfection have a more rapid rate of progression to cirrhosis than HCV monoinfected patients [1]. As death rates from opportunistic infections declined and the life expectancy is extended in the era of potent antiviral therapy in HIV infected patients, chronic hepatitis C has becomes a major cause of mortality in HIV-infected patients [2]. Marked advancements have recently been made in the antiviral treatment of HCV infection. Successful treatment of HCV infection has been shown to stop fibrosis progression, prevent liver-associated diseases and mortality in HIV-infected patients [3]. Therefore, guidelines recommend screening of all HIV-infected patients for HCV infection [4]. These recommendations also apply to patients with normal aminotransferases, since patients can have advanced fibrosis despite these laboratory findings [5]. On the other hand, because sexual transmission of HCV is not significantly frequent, routine follow-up of HCV tests in HIV-infected patients have not been recommended so far unless the patients had risk factors of HCV infection [6-10].
However, recent studies conducted in the United States, Europe, and Australia showed that incidence of HCV infection increased and the main transmission route of HCV infection was sexual contact among HIV-infected men who have sex with men (MSM) [11-14]. Increasing incidence of HCV in HIV-infected patients, combined with recent advances in antiviral therapy of HCV infection augmented the necessity of HCV screening. Therefore, the European AIDS clinical society recommended HCV testing for all of the HIV infection at time of diagnosis and thereafter annual follow-up [15]. In Japan and Taiwan, which are resource-rich and low HCV prevalent countries in Asia, increasing HCV incidence among HIV infected patients was recently reported [16,17]. Those studies also suggested routine follow-up HCV screening for HIV-infected patients. However, routine follow-up screening may not be cost effective even in a resource-rich setting if incidence of HCV in HIV-infected patients is very low. In that case, targeted screening within the high risk group could be more effective.
However, little is known about HCV epidemiology among HIV infected patients in South Korea. Therefore we designed this study to examine the incidence of HCV infection and risk factors of HCV infection among HIV infected persons in South Korea.
A retrospective cohort study was conducted from January 2000 to December 2014 at a tertiary care hospital in Pusan, South Korea. All HIV-infected patients who visited Pusan National University Hospital were identified from computerized records. To examine the prevalence and risk factors of HCV infection in HIV infected patients, patients with Ōēź 1 anti-HCV antibody tests (HCV Ab) before December 2013 were enrolled in this study. To examine the incidence of HCV in HIV infected patients, subgroup analyses were performed with the patients with a negative baseline HCV Ab who had subsequent anti-HCV Ab more than 6 months apart. Demographics and clinical data were collected from a retrospective review of medical records. The Institutional Review Board of Pusan National University Hospital approved the study protocol and informed consent was waived.
HCV Ab, CD4 cell count, HIV-1 viral load, and rapid plasma reagin (RPR) test are routinely performed on the initial visit to our institute. Patients with positive HCV Ab subsequently undergo HCV-RNA measurement and genotyping test. Repeat HCV Ab tests were performed according to the decision of the treating physician.
Prevalent HCV was defined as positive for HCV Ab at the baseline or during observation. Incident HCV was defined as positive-conversion of HCV Ab during follow-up. Clinical categories were defined by the 1993 Centers for Disease Control and Prevention classification criteria [18]. Incident syphilis, a marker of active high risk sexual behavior, was defined as a positive-conversion of RPR test or Ōēź 4-fold increase of quantitative RPR titer.
SPSS version 18.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analyses. For comparison of baseline characteristics between HCV Ab positive and negative group, Pearson chi-square test was used for categorical variables, and the Student t test for continuous variables. Multivariate logistic regression analysis was used for evaluation of the risk factors of HCV infection in HIV infected patients. Poisson regression was used for comparison of incidence rates according to periods and HIV transmission route. Tests of significance were 2-tailed, p < 0.05 was considered significant.
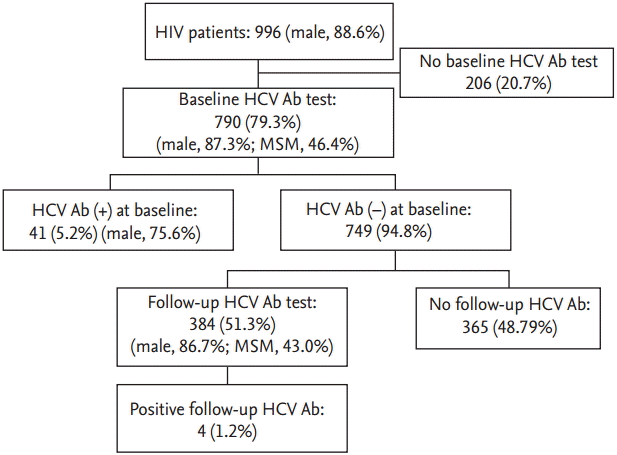
Between January 2000 and December 2013, 996 HIV-infected patients visited our hospital. Of these, 206 patients (20.7%) who had no baseline HCV Ab tests were excluded. Mean age of 790 patients (79%) with baseline HCV Ab tests was 44.0 ┬▒ 11.9 years and 690 patients (87.3%) were male including 320 (40.5%) MSM (Fig. 1).
Of 790 HIV-infected patients, 45 patients (5.7%) were positive for HCV Ab and 745 patients (94.3%) were negative. In bivariable analysis, injecting drug use (IDU), non-sexually infected HIV, female sex, and HIV RNA > 100,000 copies/mL showed significant association with prevalent HCV infection (Table 1). In multivariable analysis intravenous (IV) drug use (adjusted odds ratio, 16.20; 95% confidence interval [CI], 1.56 to 167.89; p < 0.01) was the only independent risk factor for HCV co-infection in HIV patients.
A total of 384 patients who had subsequent HCV Ab tests more than 6 months from the initial test were included for incidence analysis. Conversion to HCV Ab positivity was observed in four of 384 included patients, with an incidence rate of 2.22 (95% CI, 0.60 to 5.80)/1,000 person-years (PYs); 164.89 (95% CI, 34.00 to 481.88)/1,000 PYs in patients with IDU, 1.40 (95% CI, 0.35 to 7.79)/1,000 PYs in MSM who denied IDU, and 0 (95% CI, 0 to 3.67)/1,000 PYs in man who had sex with women (MSW) who denied IDU. Among four incident HCV patients, three were MSM and three had history of IV drug use, including two MSM and one MSW. All of the incident HCV infections developed within recent 5 years. However, there was no significant increase in incidence rate of HCV in HIV-infected MSM (p = 0.243) and total patients in 2010 to 2014 versus 2000 to 2009 (p = 0.119) (Table 2). During the study period, activity of high risk sexual behavior among enrolled patients was estimated to be constant since there was no significant change in the incidence of syphilis (Table 3).
Among the 45 HCV Ab positive patients, 21 patients were positive for HCV RNA. HCV genotype analysis was performed in 19 patients and the patients were divided into genotype 1 (n = 13, 68.4%) and 2 (n = 6, 31.6%). All of the subtype of genotype 1 was 1b. The subtypes of genotype 2 were 2 (n = 1, 5.3%), 2a/2c (n = 4, 21.1%), and mixed 2 & 2a/2c (n = 1, 5.3%). Genotype 1b infection was more prevalent in unmarried patients (1b 90% vs. non-1b 10%) and IDU (1b 6/7, 87.5% vs. non-1b 12.5%) although there was no statistical significance.
There was no significant increase of HCV incidence in South Korea. Only four HIV-infected patients were newly infected with HCV during the study period (1,551 PYs) at our hospital, with an incidence of 2.22/1,000 PYs. The incidence of 2.22/1,000 PYs was lower than that reported in North America and Western Europe and was also lower than that of resource-rich countries in Asia such as Japan and Taiwan [14,16,17,19,20]. Despite a recent minimal increase in HCV incidence in HIV-infected patients, there was no statistically significant increase over the observation periods. In addition, HCV incidence of MSM also was not significantly increased and most incident HCV patients were identified as IV drug users. Incidence of syphilis was used as a marker of ongoing sexual activity and consistent incidence of syphilis suggests that sexual activity of included patients was consistent during study periods. Although the reason for the discrepancy of HCV incidence in HIV infected patients between Korea and other resource-rich Asian countries was not clear, our data highlighted the importance of local epidemiologic data because data from other countries cannot be easily applied even though the country is geographically, racially close.
European guidelines recommended annual follow-up HCV Ab tests [15]. One recent study conducted in Japan suggested that routine rescreening for HCV is necessary among HIV-infected MSM in resource-rich settings in Asia [16]. A cost-effectiveness study which assumed a societal willingness to pay $100,000 per quality-adjusted life-year gained suggested that screening with 6-month liver function tests and a 12-month HCV Ab test was the optimal strategy when the HCV infection incidence was Ōēż 12.5 cases/1,000 PYs [21]. In our study, incidence of HCV in HIV infected patients was 2.22 PYs. Therefore annual rescreening for HCV Ab test is efficient in Korea if Korean society is willing to pay $100,000 per quality-adjusted life-year gain of HIV infected patients [21].
Our study was the first epidemiologic study on HCV infections among HIV-infected patients in South Korea. HCV prevalence among HIV-infected patients was 5.2%, higher than in the general population (0.78%) in Korea [22]. The distribution of genotype 1 and 2 was almost equal in HCV infected patients without HIV infection in Korea [22,23]. However, approximately 70% of Korean HIV/HCV co-infected patients and 87.5% of HIV/HCV co-infected patients with IDU had genotype 1b HCV. Sustained virologic response (SVR) rate of pegylated interferon ╬▒ plus ribavirin in non-HIV infected patients of 40% to 50% has been reported for genotype 1 and 70% to 80% for genotype 2 and 3 [24,25]. The benefit of antiviral therapy against HCV in HIV infected patients has been debated because of considerably low cure rates of genotype 1 HCV infection in HIV infected patients [26]. However, SVR rates of genotype 1b HCV infected patients was higher in Korea than in Western countries, which might be due to the high prevalence of IL28B-CC type among Koreans [27-29]. Furthermore, on the basis of a major breakthrough in the treatment of chronic hepatitis C using directly acting antivirals, such as telaprevir or boceprevir combined with pegylated interferon ╬▒ and ribavirin, we have entered a new era of hepatitis C therapeutics with a much higher probability of curing HCV infection including genotype 1b in HIV infected patients [30,31]. Recent studies demonstrated that use of interferon-free regimens such as sofosbuvir/ribavirin, daclatasvir/asunaprevir, and sofosbuvir/daclatasvir is comparable with interferon containing regimens in 1b HCV infected patients [32]. A better understanding of HCV epidemiology in HIV infected patients could be helpful to medical communities and government agencies in development of effective strategies for treatment of HCV.
Prevalence of HIV infected patients in South Korea is very low. In 2014, in a population of 50,220,000, there were 9,615 cumulative HIV-infected patients [33]. Approximately 14.1% of Korean HIV infected patients were notified from Busan and Gyeongsangnam-do, the Southeastern part of Korea [34]. Therefore our study included approximately 75% of HIV-infected patients in the Southeastern part of Korea and might provide useful information to understanding HIV/HCV coinfection in South Korea.
In our study, in multivariable analysis, the only risk factor for HCV infection among HIV-infected patients was experience of IV drug use.
Our study has several limitations that need to be acknowledged. First, this study was a retrospective observational study and HCV Ab were tested in clinical practice. We cannot rule out the presence of unmeasured confounding and prevent under reporting of IV drug use history. It is also possible that our data were overestimated because 41.8% of the patients with negative baseline HCV Ab test underwent follow-up HCV Ab tests and clinically suspicious patients tended to undergo follow-up HCV Ab tests. Second, our study was conducted at a single center in the southeastern region of Korea. Therefore our findings may not be generalized to other regions of the country. However, Busan could be an appropriate location for study of HCV incidence in HIV infected patients because Busan is one of the most prevalent cities for HCV infections in general populations of Korea [22].
In conclusion, this study showed that experience of IV drug use was an independent risk factor for HCV infection in HIV patients in Korea. Prevalence of HCV infection was low and incidence of HCV infection was not significantly increased in HIV-infected patients in South Korea. Further study is warranted for appropriate recommendation for routine annual follow-up of HCV Ab test in HIV-infected patients.
1. Prevalence of hepatitis C virus (HCV) in human immunodeficiency virus (HIV)-infected patients was 5.2%.
2. Intravenous drug use was independent risk factor of prevalent HCV infection in HIV-infected patients.
3. HCV incidence was 2.2/1,000 person-years and there was no significant increase of HCV incidence.
Acknowledgments
This work was supported by the clinical research grant from Pusan National University Hospital 2015.
Figure┬Ā1.
Flow diagram of included patients. HIV, human immunodeficiency virus; HCV, hepatitis C virus; Ab, antibody; MSM, men who have sex with men.
Table┬Ā1.
Comparison of characteristics between positive and negative anti-HCV Ab tests in HIV infected patients
Values are presented as mean ┬▒ SD, number (%), or median (interquartile range).
HCV, hepatitis C virus; Ab, antibody; HIV, human immunodeficiency virus; MSM, men who have sex with men; VDRL, Venereal Disease Research Laboratory test; IDU, injecting drug use; CDC, Centers for Disease Control and Prevention.
Table┬Ā2.
Incidence of hepatitis C virus infection (cases/1,000 person-years) in human immunodeficiency virus patients (95% confidence interval)
| Variable | Number | Overall period | 2000ŌĆō2009 | 2010ŌĆō2014 |
|---|---|---|---|---|
| Total | 4/384 | 2.22 (0.60ŌĆō5.68) | 0 (0ŌĆō4.04) | 3.78 (1.03ŌĆō9.67) |
| Women | 0/52 | 0 (0ŌĆō11.96) | 0 (0ŌĆō28.17) | 0 (0ŌĆō20.77) |
| Men total | 4/332 | 2.58 (0.70ŌĆō6.60) | 0 (0ŌĆō4.71) | 4.37 (1.19ŌĆō11.20) |
| ŌĆāMSM | 3/165 | 4.13 (0.85ŌĆō12.08) | 0 (0ŌĆō11.01) | 6.62 (1.36ŌĆō19.34) |
| ŌĆāMSM no drug | 1/162 | 1.40 (0.35ŌĆō7.79) | 0 (0ŌĆō11.23) | 2.23 (0.57ŌĆō12.44) |
| ŌĆāMSW | 1/167 | 1.22 (0.03ŌĆō6.81) | 0 (0ŌĆō8.31) | 2.18 (0.06ŌĆō12.20) |
| ŌĆāMSW no drug | 0/166 | 0 (0ŌĆō3.67) | 0 (0ŌĆō8.31) | 0 (0ŌĆō6.57) |
| IDUa | 3/4 | 164.89 (34.00ŌĆō481.88) | 0 (0ŌĆō367.11) | 299.25 (61.71ŌĆō874.54) |
Table┬Ā3.
Incidence of syphilis (cases/1,000 person years) in human immunodeficiency virus patients (95% confidence interval)
REFERENCES
1. Fierer DS, Dieterich DT, Fiel MI, et al. Rapid progression to decompensated cirrhosis, liver transplant, and death in HIV-infected men after primary hepatitis C virus infection. Clin Infect Dis 2013;56:1038ŌĆō1043.



2. Sulkowski MS, Thomas DL. Hepatitis C in the HIV-infected person. Ann Intern Med 2003;138:197ŌĆō207.


3. Berenguer J, Alvarez-Pellicer J, Martin PM, et al. Sustained virological response to interferon plus ribavirin reduces liver-related complications and mortality in patients coinfected with human immunodeficiency virus and hepatitis C virus. Hepatology 2009;50:407ŌĆō413.


4. The U.S. Department of Health and Human Services (DHHS). Guidelines for the Use of Antiretrovial Agents in HIV-1-infected Adults and Adolescents [Internet]. Rockville (MD): AIDSinfo, 2014. [cited 2016 Mar 30]. Available from: https://aidsinfo.nih.gov/guidelines.
5. Bani-Sadr F, Barange K, Daoud F, et al. Persistently normal alanine aminotransferase levels in HIV/HCV-coinfected patients: the role of steatosis. HIV Med 2009;10:417ŌĆō421.


6. Kao JH, Liu CJ, Chen PJ, Chen W, Lai MY, Chen DS. Low incidence of hepatitis C virus transmission between spouses: a prospective study. J Gastroenterol Hepatol 2000;15:391ŌĆō395.


7. Murphy EL, Bryzman SM, Glynn SA, et al. Risk factors for hepatitis C virus infection in United States blood donors. NHLBI Retrovirus Epidemiology Donor Study (REDS). Hepatology 2000;31:756ŌĆō762.


8. Rauch A, Rickenbach M, Weber R, et al. Unsafe sex and increased incidence of hepatitis C virus infection among HIV-infected men who have sex with men: the Swiss HIV Cohort Study. Clin Infect Dis 2005;41:395ŌĆō402.


9. Vandelli C, Renzo F, Romano L, et al. Lack of evidence of sexual transmission of hepatitis C among monogamous couples: results of a 10-year prospective follow-up study. Am J Gastroenterol 2004;99:855ŌĆō859.


10. Soto B, Rodrigo L, Garcia-Bengoechea M, et al. Heterosexual transmission of hepatitis C virus and the possible role of coexistent human immunodeficiency virus infection in the index case: a multicentre study of 423 pairings. J Intern Med 1994;236:515ŌĆō519.


11. Browne R, Asboe D, Gilleece Y, et al. Increased numbers of acute hepatitis C infections in HIV positive homosexual men: is sexual transmission feeding the increase? Sex Transm Infect 2004;80:326ŌĆō327.



12. Danta M, Brown D, Bhagani S, et al. Recent epidemic of acute hepatitis C virus in HIV-positive men who have sex with men linked to high-risk sexual behaviours. AIDS 2007;21:983ŌĆō991.


13. Taylor LE, Holubar M, Wu K, et al. Incident hepatitis C virus infection among US HIV-infected men enrolled in clinical trials. Clin Infect Dis 2011;52:812ŌĆō818.



14. van de Laar TJ, van der Bij AK, Prins M, et al. Increase in HCV incidence among men who have sex with men in Amsterdam most likely caused by sexual transmission. J Infect Dis 2007;196:230ŌĆō238.


15. European AIDS Clinical Society. European guidelines for treatment of HIV infected adults version 7.1 [Internet]. Brussels (BE): European AIDS Clinical Society, c2016. [cited 2016 Mar 30]. Available from: http://www.eacsociety.org.
16. Nishijima T, Shimbo T, Komatsu H, Hamada Y, Gatanaga H, Oka S. Incidence and risk factors for incident hepatitis C infection among men who have sex with men with HIV-1 infection in a large urban HIV clinic in Tokyo. J Acquir Immune Defic Syndr 2014;65:213ŌĆō217.


17. Sun HY, Chang SY, Yang ZY, et al. Recent hepatitis C virus infections in HIV-infected patients in Taiwan: incidence and risk factors. J Clin Microbiol 2012;50:781ŌĆō787.



18. 1993 Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep 1992;41(RR-17):1ŌĆō19.

19. Garg S, Taylor LE, Grasso C, Mayer KH. Prevalent and incident hepatitis C virus infection among HIV-infected men who have sex with men engaged in primary care in a Boston Community Health Center. Clin Infect Dis 2013;56:1480ŌĆō1487.



20. Giraudon I, Ruf M, Maguire H, et al. Increase in diagnosed newly acquired hepatitis C in HIV-positive men who have sex with men across London and Brighton, 2002-2006: is this an outbreak? Sex Transm Infect 2008;84:111ŌĆō115.


21. Linas BP, Wong AY, Schackman BR, Kim AY, Freedberg KA. Cost-effective screening for acute hepatitis C virus infection in HIV-infected men who have sex with men. Clin Infect Dis 2012;55:279ŌĆō290.



22. Kim do Y, Kim IH, Jeong SH, et al. A nationwide seroepidemiology of hepatitis C virus infection in South Korea. Liver Int 2013;33:586ŌĆō594.


23. Oh DJ, Park YM, Seo YI, Lee JS, Lee JY. Prevalence of hepatitis C virus infections and distribution of hepatitis C virus genotypes among Korean blood donors. Ann Lab Med 2012;32:210ŌĆō215.



24. Park SH, Park CK, Lee JW, et al. Efficacy and tolerability of peginterferon alpha plus ribavirin in the routine daily treatment of chronic hepatitis C patients in Korea: a multi-center, retrospective observational study. Gut Liver 2012;6:98ŌĆō106.



25. Afdhal NH, McHutchison JG, Zeuzem S, et al. Hepatitis C pharmacogenetics: state of the art in 2010. Hepatology 2011;53:336ŌĆō345.


27. Jeong SH, Jung YK, Yang JW, et al. Efficacy of peginterferon and ribavirin is associated with the IL28B gene in Korean patients with chronic hepatitis C. Clin Mol Hepatol 2012;18:360ŌĆō367.



28. Jung YK, Kim JH, Ahn SM, et al. Role of interleukin 28B-related gene polymorphisms in chronic hepatitis C and the response to antiviral therapy in Koreans. J Clin Gastroenterol 2013;47:644ŌĆō650.


29. Kil H, Jeong SH, Kim JW, et al. Role of interleukin-28B genetic polymorphisms in Korean patients with hepatitis C virus infection. Gut Liver 2014;8:70ŌĆō78.



30. Sulkowski MS, Sherman KE, Dieterich DT, et al. Combination therapy with telaprevir for chronic hepatitis C virus genotype 1 infection in patients with HIV: a randomized trial. Ann Intern Med 2013;159:86ŌĆō96.


31. Sulkowski M, Pol S, Mallolas J, et al. Boceprevir versus placebo with pegylated interferon alfa-2b and ribavirin for treatment of hepatitis C virus genotype 1 in patients with HIV: a randomised, double-blind, controlled phase 2 trial. Lancet Infect Dis 2013;13:597ŌĆō605.


32. Holmes JA, Thompson AJ. Interferon-free combination therapies for the treatment of hepatitis C: current insights. Hepat Med 2015;7:51ŌĆō70.



33. Korean Centers for Disease Control and Prevention. Annual report on the notified HIV/AIDS in Korea 2014 [Internet]. Cheongju (KR): Korean Centers for Disease Control and Prevention, 2014. [cited 2016 Mar 30]. Available from: http://nih.go.kr/NIH_NEW/main.jsp.
34. Korean Centers for Disease Control and Prevention. Annual report on the notified HIV/AIDS in Korea 2010 [Internet]. Cheongju (KR): Korean Centers for Disease Control and Prevention, 2010. [cited 2016 Mar 30]. Available from: http://nih.go.kr/NIH_NEW/main.jsp.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



