INTRODUCTION
Many different organisms from phytoplankton to vertebrate animals synthesize vitamin D, a lipid soluble molecule usually produced after exposure to solar ultraviolet rays. In 1822, Sniadecki described this vitamin and associated its function to musculoskeletal system homeostasis, when he correlated rickets found in children with little exposure to sunlight from industrialized cities [1,2].
In the last decades, an increasing interest has arisen regarding the role that vitamin D plays in the metabolism of the different human organs and systems. There is a complex biologic interaction between this molecule and the organism; namely, its receptors are involved in several signalling pathways, and are not only related to calcium metabolism [3,4].
It is estimated that 30% to 50% of worldwide population suffers a form of vitamin D deficiency. This has been proven to be more frequent in populations living more distant to the equatorial line, resulting in important repercussions to their well-being and health [4]. The connection between the lack of vitamin D and a number of diseases is well established. Some of these diseases are cancer [5], psoriasis [6], asthma [7], rheumatoid arthritis [8], multiple sclerosis [9], inflammatory bowel disease [10], insulin resistance [11], type 2 diabetes mellitus (T2DM), metabolic syndrome, obesity, hypertension, and cardiovascular disease [4].
Despite the fact that several studies have been published on this topic, knowledge about the relation between vitamin D and cardiovascular disease is still lacking, for the detriment of healthcare professionals. This review presents the most relevant and most recent published information on the subject, referring to a wide variety of molecular, animal, and human studies. In this article, we have also taken into account the different aspects, in which vitamin D is presumably involved with the various aspects of cardiovascular diseases. Therefore, the aim of this article is to elucidate to physicians the different associations and mechanisms regarding vitamin D and cardiovascular disease, with an objective perspective.
MATERIAL AND METHODS
In this review, we conducted an exhaustive search in Scopus, EBSCO, and PubMed and looked for the most recent evidence in articles about vitamin D and cardiovascular system.
Metabolism
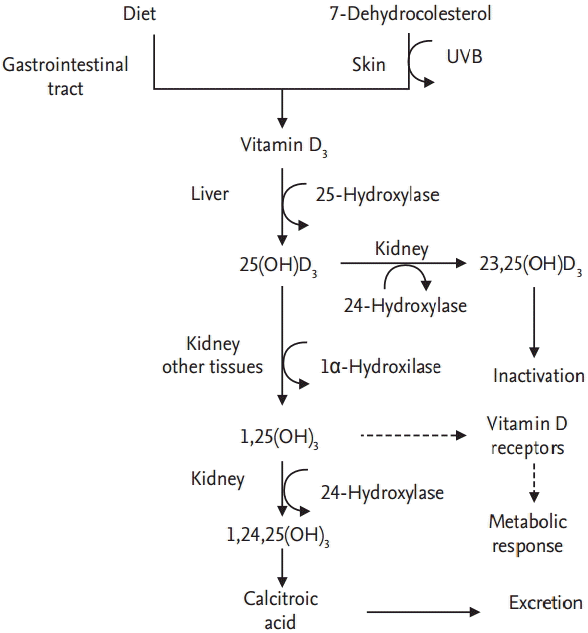
Vitamin D3 (cholecalciferol) is a steroid hormone found in nature, and can be acquired from various sources [12], including cow milk, eggs, fish (salmon and tuna), and eel. It is absorbed by the gastrointestinal tract. In addition, 7-dehydrocholesterol, a serum circulating cholesterol precursor, is converted to cholecalciferol in the skin when exposed to ultraviolet B (UVB, 280 to 315 nm) and carried to the liver by the vitamin D-binding protein (Gc-globulin). Then, it is hydroxylated on position C-25 into 25-hydroxyvitamin D (calcifediol, 25(OH)D, or 25(OH)D3) by the 25-hydroxylase, an enzyme located in the cytochrome P450 (CYP2R1, CYP2D11, and CYP2D25).
The 25(OH)D is a major metabolite circulating in blood. It is transported by vitamin D-binding protein to the kidneys, where it is transformed into 1,25-dihydroxyvitamin D (calcitriol, 1,25(OH)D or 1,25(OH)2D3) by the 1öÝ-hydroxylase enzyme of the proximal convoluted tubule. 1,25(OH)D destined for excretion is finally metabolized in the kidneys, where it is transformed initially into 24,25(OH)2D3, next into 1,24,25(OH)2D3 (1,24,25-dihydorxyvitamin D) and finally into calcitroic acid, which is then excreted through urine (Fig. 1) [13-16].
Since this enzyme is present in various tissues, such as placenta, monocytes, and macrophages, the aforementioned process is not exclusive of the kidneys. 1,25(OH)2D3 is responsible for most of vitamin D metabolic actions, including those related to calcium [13,14].
Apart from the classical metabolic action of 1,25(OH)2D3 over calcium, phosphate and the parathyroid hormone (PTH), other complex metabolic actions are mediated by vitamin D receptors (VDRs). VDRs are intracellular receptors which bind 1,25(OH)2D3, and are found in over 36 tissues, including vascular smooth muscle, endothelium, and myocardium. Interestingly, VDRs play a crucial role in the homeostasis, hence actively regulating the cardiovascular system [13-17].
Serum levels variation and its risk factors
Variations in serum levels of the vitamin D metabolites have many clinical implications, and different scales to classify these levels have been suggested [18-20]. The Institute of Medicine concluded that 25(OH)D serum levels of 16 ng/mL covers the requirements of approximately 50% of the population, 25(OH)D serum levels of 20 ng/mL covers the requirements of ãË 97.5% of the population, while 25(OH)D levels of 50 ng/mL or greater should raise concerns about potential adverse effects [21]. On the other hand, the U.S. Endocrine Society guidelines defines vitamin D deficiency as 25(OH)D less than 20 ng/mL (50 nmol/L), vitamin D insufficiency as a 25(OH)D level between 21 and 29 ng/mL, and the safety margin to minimize the risk of hypercalcemia as a 25(OH)D level equal to 100 ng/mL (250 nmol/L) [22].
Several risk factors may condition and predispose to vitamin D deficiency, for instance, low exposure to sunlight, skin hyperpigmentation, low dietary intake, smoking, pollution, aging, sedentarism, intestinal malabsorption, kidney disease, liver disease, and deficiency secondary to medication and genetic factors [20,23,24]. Obesity is associated with low serum 25(OH)D levels, which could be the result of several mechanisms. One hypothesis suggests that a high content of body fat acts as a reservoir for lipid soluble vitamin D. This increases its sequestration, and evokes a low bioavailability. Other hypotheses suggest that higher leptin and interleukin 6 (IL-6) circulating levels, mostly secreted by adipose tissue, may have inhibitory effects on 25(OH)D synthesis via their receptors [25].
Coronary artery disease and other vascular diseases
Coronary artery disease (CAD) remains the main cause of death in developing countries. It is a disease of multifactorial etiology in which metabolic, genetic, hemodynamic, and inflammatory factors are involved [26,27]. Just as in other cardiovascular diseases, it has been established that vitamin D plays an active role in CAD through different mechanisms, as elucidated next [28].
In animal models, it has been established that vitamin D plays an active role in arterial hardening and endothelial function, mediated by both VDRs and 1öÝ-hydroxylase found in these tissues. Endothelial dysfunction is caused by a reduction in nitric oxide bioavailability in VDR deficient mice [29]. In addition, vitamin D makes a counter regulatory process in the renin-angiotensin-aldosterone system (RAAS) by diminishing its proliferating effects on the vascular smooth muscle cells, and reducing CAD [30].
Research has shown that vitamin D protects vessel walls against damage caused by inflammation by increasing the expression of anti-inflammatory cytokines, such as IL-10, and by decreasing expression of pro-inflammatory molecules, e.g., tumor necrosis factor öÝ (TNF-öÝ) and IL-6 [31]. Vitamin D is also involved in the down-regulation of plaque-destabilizing enzymes, like matrix metalloproteinase 9 (MMP-9) [32], as well as in the C-reactive protein elevation. Yet, the involvement of the latter is not supported by recent studies [33].
In vitro models, using human umbilical cord endothelial cells, suggest that vitamin D may be useful in treating acute coronary syndromes by reducing VCAM-1 (vascular cellular adhesion molecule), MMP-1, and CD62P (P-selectin) expression, and indirectly regulating platelet aggregation [34]. Vitamin D is also now considered a prognostic factor for acute myocardial infarction and CAD [32,35-37]. In addition, Jablonski et al. [38] found endothelial dysfunction in adults with low 25(OH)D3, as opposed to patients with normal levels, through the measurement of nuclear factor-ö¤B (0.59 ôÝ 0.07 vs. 0.44 ôÝ 0.05, p < 0.05) and other pro-inflammatory cytokines in endothelial cells.
Accordingly, vitamin D contributes to vascular structure, function and remodeling, as well as to the pathophysiology of endothelial dysfunction and atherosclerosis. Low vitamin D serum levels have been identified as a potential risk factor for death, (odds ratio [OR], 1.16; 95% confidence interval [CI], 1.012 to 1.334; p = 0.03) [39-41]. To demonstrate this, Seker et al. [42] compared 69 adults with vitamin D deficiency to a group of 62 healthy non-vitamin D-deficient people. They calculated pulse wave velocity in both groups, finding that it was statistically higher (p < 0.02) in the vitamin D deficient group, indicating vascular stiffness and endothelial dysfunction. These findings match those of Al Mheid et al. [43], which connect vitamin D deficiency to vascular disease.
Syal et al. [44] reported that severe CAD (double or triple vessel CAD) was more frequent in patients with 25(OH)D levels < 20 ng/mL as compared to those with levels > 20 ng/mL (53% vs. 38%, p = 0.03). Diffuse CAD on coronary angiography was also more frequently present in those with lower 25(OH)D levels (56% vs. 38%, p = 0.03). In addition, the mean number of coronary vessels involved was also higher in those with lower 25(OH)D levels (1.78 ôÝ 0.76 vs 1.24 ôÝ 0.43, p = 0.05) [44]. Chen et al. [45] proposed that low vitamin D levels are associated with the severity of coronary artery stenosis and Joergensen et al. [46] postulated that diabetic patients with vitamin D deficiency have a higher risk to develop CAD (OR, 2.9; 95% CI, 1.02 to 7.66). In addition, it has been reported that HIV positive African-Americans with a vitamin D deficiency have a higher risk for presenting coronary artery stenosis (OR, 2.28; 95% CI, 1.23 to 4.21) [47].
Vitamin D also plays a role in vascular calcification, a multifactorial process with deep clinical implications [48,49]. Vitamin D regulates PTH, which in turns mediates calcium metabolism; vascular calcification is a common complication when this pathway is modified. In addition, vitamin D interacts with fibroblast growth factor 23, Klotho enzyme, phosphate, and a large range of immunological and metabolic pathways. Vascular calcification is related to all these factors, and is therefore linked to vitamin D [13,50,51]. Randomized studies in low density lipoprotein (LDL) receptor knockout (LDLRã/ã) mice fed with a low vitamin D diet, have demonstrated enhanced vascular calcification. This is probably related to the observation that a low vitamin D diet modifies expression of vascular osteoblast differentiation factors and verifiable osteoblast-like cells [52]. However, high-dose calcitriol treatment induces an osteoblastic phenotype in vascular smooth muscle cells, both in subtotally nephrectomized rats and in vitro. This effect is associated with an up-regulation not only of proteins regulating mineralization and calcium transport, but also of osterix (osteogenic transcription factor) [53].
A small study in apolipoprotein E-deficient mice has postulated a novel cardioprotective role for VDRs in circulating endothelial cells. In this study, authors suggest that VDR deficiency not only promoted the development of atherosclerosis, but also decreased the stability of atherosclerotic plaques. This happens through mechanisms that include cell proliferation and differentiation, apoptosis, oxidative stress, membrane transport, matrix homeostasis, and cell adhesion. Additionally, they suggest that VDRs up-regulates eNOS (endothelial nitric oxide synthase) protein expression, an important enzyme that contributes to the pathogenic process of atherosclerosis [54].
Interestingly, just like vitamin D deficiencies, hypervitaminosis or a state of toxicity will lead to vascular calcification. This observation was made in dialysis patients where a U-shape curve has been noticed, where low serum vitamin D levels, as well as high levels, are associated with vascular calcification [13,50,51]. However, other authors have postulated that there is insufficient evidence to support a consistent association between low vitamin D levels and coronary artery calcium (CAC) [55], or that there is definitely no association between low 25(OH)D and CAC, or severely obstructive coronary artery stenosis [56]. Nevertheless, single nucleotide polymorphisms (SNPs) in the CYP24A1 gene, associated with vitamin D metabolism, have been independently linked with coronary artery calcification [57]. For this reason, future studies should analyze the results in the light of the genetic population variability, taking into account the SNPs that affect the VDRs or enzymes that are related with vitamin D metabolism. These studies should also evaluate serum 25(OH)D and its relationship to subclinical coronary atherosclerosis.
Indeed, vitamin D deficiency has also been identified as a potential risk factor for peripheral artery disease (OR, 1.35; 95% CI, 1.15 to 1.59) [58], if 25(OH)D3 levels decrease below 10 ng/mL [59]. In addition, Chaudhuri et al. [60] estimated that 25(OH)D3 deficiency may also be a risk factor for ischemic stroke (OR, 1.6; 95% CI, 1.2 to 2.8), concurring with other authors who report 25(OH)D3 levels as prognostic and severity predictors in patients with ischemic stroke [61].
Hypertension
Systemic arterial hypertension is a severe public health issue [62]. Hypertension is considered an isolated cardiovascular risk factor; however, it is associated with CAD, stroke, and renal failure [63,64], and has a multifactorial etiology. Even more, it has been demonstrated that RAAS plays a major role in hypertension pathophysiology, affecting sodium reabsorption in the kidney, as well as vasculature reactivity [65].
A recent meta-analysis has postulated that vitamin D is involved in this disease, showing that patients with low 25(OH)D3 serum levels may have more risk to develop hypertension compared to those with normal parameters (OR, 1.37; 95% CI, 1.19 to 1.59) [66]. It is believed that this molecule takes part in the process via different mechanisms, but its effect over RAAS, as mentioned above, is one of the most important [67]. Animal models show that calcitriol binds to the promoter region in the REN-1C gene and thus suppresses renin expression, lowering the risk to develop hypertension. Furthermore, renal arteries in vitamin D deficient individuals have lower expression of angiotensin-l receptors, when they are exposed to the molecule [68]. Studies demonstrate that VDR and 1öÝ-hydroxylase knockout mice develop hypertension due to a higher expression of renin [69,70]. They also report that vitamin D deficient diets predispose to an accelerated activation of RAAS with subsequent hypertension and atherosclerosis [71]. Hence, vitamin D could be a cardiovascular protection factor.
Another mechanism through which low levels of vitamin D may exert influence to hypertension generation, is by direct interaction with the endothelium and vasculature [72]. Chronic treatment with calcitriol has shown that hypertensive rats have decreased endothelium dependent vascular contraction, reduced quantities of reactive oxygen species, and diminished expression of cyclooxigenase-1 [73], which suggests that vitamin D directly confers a protective function against endothelial dysfunction [74].
A third mechanism suggested in the implication of vitamin D and hypertension is regarding PTH and calcium metabolism. Hypovitaminosis D brings about an increment in PTH secretion, which will, at first, relax the vasculature and will eventually constrict it, thereby causing hypertension. Pathophysiologically, this will happen due to an increase of intracellular calcium levels, resulting in endothelial and vascular growth dysfunction [75]. Population studies also support these theories [76-78]. Rostand [79] postulated that the farther a population is settled from the equatorial line, the higher its blood pressure will be, estimating that for every 10 degrees of distance, pressure will rise 2.5 mmHg. This suggests that the relationship is established based on exposure to sunlight and consequent vitamin D availability. Forman et al. [80] showed that angiotensin-II levels are statistically different (p = 0.03) in individuals with regular calcifediol levels compared to those with insufficient to deficient levels.
Additionally, in their double-blind study, Nasri et al. [81] showed that supplementary therapy with cholecalciferol based on 50,000 units weekly for 12 weeks, reduces blood pressure significantly (p < 0.01) in diabetic patients. This result is line with the data presented by Larsen et al. [82], who reported that a daily supplementation of 3,000 units of cholecalciferol reduces central systolic pressure (p = 0.007). Although vitamin D supplementation has not been proven as a beneficial treatment for hypertension in all ethnic and age groups [81], Forman et al. [83] suggested that it is indeed beneficial, specifically in the case of the African-American population. Similarly, Judd et al. [84] observed in a case-control study that patients under calcitriol supplement treatment (0.5 ö¥g twice daily) have a statistically significant lower blood pressure (p < 0.001). Nonetheless, it has been shown that there is no benefit in vitamin D supplementation in geriatric population with isolated systolic hypertension [85].
Even though one meta-analysis has shown that, in patients with the diagnosis of hypertension, supplementary vitamin D can reduce the diastolic blood pressure ã3.1 mmHg (95% CI, ã5.5 to ã0.6) and the systolic blood pressure ã6.2 mmHg (95% CI, ã12.32 to ã0.04), supplementary vitamin D has no effect in patients without hypertension [86]. Other authors support the hypothesis that vitamin D supplementation is ineffective to lower blood pressure in the general population, and thus advice against its use as an antihypertensive agent [87,88].
Regarding pulmonary hypertension, Demir et al. [89] estimated that there is a statistically significant difference p < 0.001) in the systolic pulmonary artery pressure in patients with low levels of vitamin D (36.31 ôÝ 8.99 mmHg) and those with adequate serum levels (32.42 ôÝ 8.06 mmHg). This promising result notwithstanding, currently there is not enough evidence to correlate pulmonary hypertension and vitamin D levels abnormalities.
Heart failure and vitamin D
Worldwide, heart failure (HF) represents a major health problem with an incidence of 1% to 2%. It is considered a complex disease with multiple causes. Actually, vitamin D and its metabolic pathway have been described as related agents for HF, both in the development and progression of the disease [90,91].
In rats, vitamin D is a protective factor in the development of ventricular hypertrophy and cardiac dysfunction [92]. Alternatively, studies in swine suggest that vitamin D deficiency, diminished expression of both VDRs and the suppressor of cytokine signaling 3 (SOCS3) gene are associated to myocardial hypertrophy [93].
In a clinical trial, Shedeed [94] studied 80 children with congestive HF and divided them into two groups; the first one received vitamin D supplementation for 12 weeks, and the second one received a placebo. The evaluation at the end of the period reports a significant improvement in the former group regarding the ejection fraction of the left ventricle, as well as an increase in IL-10 and a decline in PTH, IL-6, and TNF-öÝ [94]. This might be explained by the inhibitory processes that vitamin D plays in RAAS [95]. In addition, after studying a large population, Ford et al. [96] suggested that vitamin D supplementation might protect against cardiac failure in older people. Furthermore, it has been postulated that serum levels of 25(OH)D may be a marker and a modulator of the functional capacity in HF of the elderly [97].
Atrial fibrillation
Atrial fibrillation (AF) is estimated to have a prevalence of 2% in the world population and has multifaceted implications in health and quality of life [100]. Valvular abnormalities, hypertension, and diabetes have been associated to AF. Actually, genetics and molecular pathways are deeply involved in the development of the disease [101].
It was not until 1990, when Kessel [102] described the possible correlation between vitamin D and AF. Even though the exact connection is still unknown, we can hypothesize a correlation between them from two facts: calcitriol contributes to smooth muscle proliferation and affects the RAAS, and angiotensin II levels are above normal in AF [103]. Some literature suggests that 25(OH)D3 levels are deficient in patients diagnosed with AF [104,105]. By way of contrast, it has been reported that individuals with 25(OH)D levels higher than 100 ng/mL have a greater risk to suffer from AF [106]. And yet, other studies have shown that there is no correlation between vitamin D and AF [107,108]. The current evidence is consequently insufficient to sustain the correlation between AF and vitamin D.
Insulin resistance
Vitamin D status is inversely associated with total body fat. A higher storage in adipose tissue is a plausible explanation for increased rates of deficiency in obese patients [109]. Indeed, an important correlation between vitamin D deficiency and metabolic syndrome risk factors has been established [110].
Increased insulin resistance is found associated with low vitamin D status in a variety of observational studies, for instance, population-based studies in geographically diverse countries [111,112]. Hypponen and Power [109] reported a statistically significant negative association between vitamin D status and hemoglobin A1c. Further, von Hurst et al. [113] proved that improving vitamin D status in insulin resistant patients resulted in ameliorated insulin resistance and sensitivity without change in insulin secretion.
Despite the previous observations, some authors have suggested that the association between Vitamin D and insulin resistance is presumably coincidental [112]. In addition, a recent meta-analysis by Wallace et al. [114] suggests that it is unlikely that vitamin D supplementation has any effect on T2DM incidence or insulin resistance, and could be a surrogate marker whose attenuation may not be clinically relevant.
VITAMIN D AND MOLECULAR BIOLOGY
VDRs, found on both the cell and nuclear membranes [3,4], have an influence through genomic and non-genomic pathways in the function and structure of cardiomyocytes and cardiovascular system cells [115,116]. It has also been reported that within this broad group of receptors, certain polymorphisms can be used as predictors for developing left ventricular hypertrophy in patients with end stage kidney disease [117] or other diseases, such as Gaucherãs variant heart disease [118]. In a study of 293 individuals with angina and hypercholesterolemia, Ortlepp et al. [119] established that the genotype of the VDR is crucial for the prevalence of T2DM and EAC.
Certain allelic variants of the vitamin D carrier protein have been associated with small changes in serum levels of this vitamin. Therefore, it is possible that the levels of vitamin D and their consequent systemic action may vary according to the genetic characteristics of populations. Hence, we can deduce that the impact to the organs and systems, including the cardiovascular system, will depend in part on the genotype of each patient [120].
We consider important to determine the relationship between certain polymorphisms of genes associated with VDRs [121], which could modify their spatial conformation and their expression in their signaling pathways. Alternatively, the genomics and the proteomics of all components of the metabolic pathway of vitamin D (enzymes and vitamin D binding protein) should be analyzed to reveal the degree to which vitamin D level changes affects the cardiovascular system. In fact, genetic variability may explain the ambivalence in literature, since studies were conducted on specific ethnic groups.
CONCLUSIONS
Vitamin D is actively involved in multiple metabolic pathways, including those involved in the homeostasis of the cardiovascular system. Low vitamin D levels have been identified as a risk factor for several major cardiovascular diseases [122], including hypertension and pulmonary hypertension, atherosclerosis, CAD, vascular insufficiency, stroke, HF, and AF. Despite the presented information, evidence about these associations remains ambivalent, since a large number of results generated by basic studies and animal models are in contrast with the information obtained in randomized controlled studies. In the current state of knowledge, it may be too early to consider vitamin D as an instrument to either estimate or mitigate residual cardiovascular risk. For this reason, in our opinion, further studies should assess the role of vitamin D in relation with different pathologies; in the context of the variability of the genes that code for VDRs, enzymes and metabolites, since they directly interact with metabolic pathways related to this vitamin. Nevertheless some of these pathways may still need to be elucidated.
We consider that it is necessary to continue exploring the cardiovascular effect of vitamin D, and to analyze the associations and risks related to changes in the levels of this molecule in different populations. This information may provide improved clinical guidelines, which would indicate the need to monitor the levels of this vitamin in the general population, in some ethnicities or specific groups, regarding these pathologies. However, it is unclear whether vitamin D supplementation can reduce cardiovascular risk or mitigate cardiovascular disease. Therefore, we consider that this molecule should not be recommended for this indication to the general population.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





