 |
 |
| Korean J Intern Med > Volume 33(4); 2018 > Article |
|
Abstract
Background/Aims
We explored Korean physicians’ policies for surveillance of colorectal cancer (CRC) after curative surgery.
Methods
Web-based self-report questionnaires were developed. Invitations to participate were emailed to physicians who diagnosed and treated CRC from October 1 to November 15, 2015. The questionnaire consisted of the role doctors played in the surveillance, examination of surveillance, and duration of postoperative surveillance according to CRC stage or primary site of the cancer.
Results
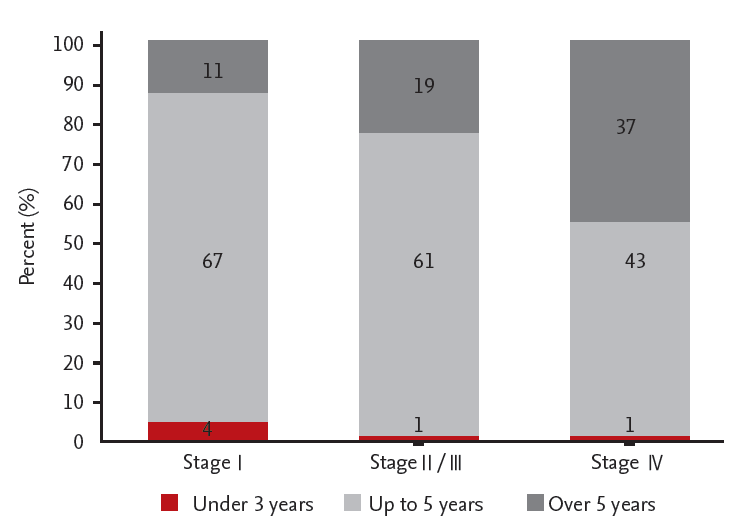
Ninety-one physicians participated in the online survey, and 78 completed the survey. Sixty-seven participants (13%) answered “up to 5 years” for stage I surveillance duration; and 11 (13%) responded with a duration of > 5 years for stage I. A total of 61 (75%) responded with a surveillance duration of up to 5 years for stage II; and 19 (24%) responded with a duration of > 5 years for stage II. Sixty-seven (97%) and 61 (91%) physicians monitored patients with stage II/III every 3 or 6 months by laboratory examination and by abdominopelvic computed tomography scan for the first year, respectively. A total of 43 (53%) responded with a surveillance duration of up to 5 years for stage IV; and 46 (46%) responded with a duration of > 5 years for stage IV after curative resection.
Conclusions
Korean physicians mostly followed up CRC using intensive postoperative surveillance. In preference to monitoring over a comparatively shorter period of time, the physicians tended to prefer monitoring patients post-operatively over a > 5 year period, particularly in cases of advanced-stage CRC.
The incidence of colorectal cancer (CRC) in Korea has increased. Annual percentage changes in age-standardized incidence rates were 5.7% in men and 4.3% in women between 1999 and 2012, using the world standard population. In 2012, 28,988 new CRC cases (17,445 men and 11,543 women) were diagnosed, accounting for 13.0% of all CRC cases [1]. Although the prognosis mainly depends on tumor stage at diagnosis, early treatment upon relapse is critical to improve patients’ prognosis [2,3]. A small portion of CRC cases with isolated metastasis, such as liver or lung metastasis, can be cured with surgery.
Surveillance after curative resection can be expensive and resource-consuming, in terms of both cost and procedures; so intensive surveillance must be justified with a good level of evidence. Previous studies have shown that 80% of CRC recurrences occur during the first 3 years after surgical resection of the primary tumor [4], and about 90% of recurrences occur within the first 5 years [5]. Most follow-up programs end 5 years after the initial curative surgery, and the usefulness of postoperative follow-up has been controversial. Surveillance policies vary widely according to centers and countries. Moreover, the surveillance interval and duration vary according to stage and primary site.
Different specialist physicians—such as medical oncologists, surgeons, gastroenterologists, and radiation oncologists—are responsible for cancer surveillance in Korea. We explored the current role of physicians in surveillance and physicians’ policies for surveillance of CRC after curative surgery in Korea.
We conducted this survey prospectively to explore postoperative surveillance: a guideline review survey in Korea, which was a symposium hosted by a multidisciplinary education program to demonstrate the current practices of doctors who play a role in surveillance after curative treatment. This study was performed from October 1 to November 15, 2015. The survey instrument was developed by the Chemotherapy Study Committee of the Korean Society of Coloproctology (KSC), the Colorectal Cancer Study Committee of the Korean Society of Gastrointestinal Cancer (KSGC), and the Colorectal Cancer Committee of the Korean Cancer Study Group (KCSG). The KSC, KSGC, and KCSG sent emails to their members that included the online survey website. All respondents, prior to participation, agreed to allow the use of their responses for the analysis in the present study.
We collected physicians’ clinical data, including doctor specialty and monthly mean number of patients consulted with CRC. The questionnaire consisted of the roles doctors play in surveillance (roles such as surgeon, medical oncologist, gastroenterologist, radiation oncologist, and others), surveillance examinations, and interval or duration according to stage or primary site (rectum vs. colon) after curative treatment. For surveillance of CRC after curative surgery, stage IV was limited to those subjects deemed capable of receiving curative resection. The questionnaire asked what tests had been included in follow-up examinations—such as complete blood count (CBC), chemistries, chest computed tomography (CT) scans, tumor markers, colonoscopy, abdominopelvic CT, bone scan, and positron emission tomography (PET) scans after curative surgery. The questionnaire asked whether laboratory examinations, chest CTs, abdominopelvic CTs, and PET scans occurred in every 3-month, 6-month, or 1-year intervals. Bone scans were likewise reportable as typically being performed in every 6-month, 1-year, 2-year, or 3-year intervals. The questionnaire also asked whether colonoscopy was performed during the first 6 months, 1st year, 2nd year, 3rd year, and/or 5th year following surgery. If multiple colonoscopies were performed, the questionnaire allowed physicians to select multiple options.
Descriptive analyses of the clinical data and CRC surveillance practices—such as the physicians’ role, duration or interval of follow-up, and laboratory and imaging study schedules—were performed.
Ninety-one doctors participated in the online survey, and 78 completed the questionnaire. Of the 78 doctors in 72 hospitals, 39 (50%) were medical oncologists, 24 (31%) were surgeons, and 15 (19%) were gastroenterologists. More than 85% of the doctors treated ≥ 10 patients with CRC per month.
For duty on physician according to stage, medical oncologists were responsible for the surveillance of 6% of patients with stage I and 69% of postoperative patients with stage IV CRC. Surgeons and gastroenterologists followed up with 93% and 27% of patients with stage I and 54% and 17% of patients with stage IV, respectively (Table 1). Radiation oncologists participated to a small degree of the postoperative surveillance of patients with stages II/III CRC.
Four participants (5%) answered that patients with stage I are followed up with for 3 years; 67 (82%) stated 5 years; and 11 (13%) stated > 5 years. Sixty-one physicians (75%) answered that patients with stage II/III need to be followed up with for 5 years; and 19 (24%) stated that these patients need to be followed up with for > 5 years. Forty-three physicians (53%) reported having monitored patients with stage IV for 5 years; and 37 (46%) reported having monitored these patients for > 5 years (Fig. 1). Seventy-six physicians (89%) answered that there was no difference between total surveillance duration according to location (colon vs. rectum); and nine (11%) said that there was such a difference in duration.
Thirty-five participants (40%) answered that the surveillance time interval was the same regardless of cancer stage; and 52 (60%) answered that it was different. Forty-four participants (51%) answered that there was no difference in the surveillance time interval for patients with stages I and II/III, and 43 (49%) said that there was a difference. Forty participants (45%) answered that the surveillance time interval between patients with stages II/III and IV was the same; and 48 (55%) gave the answer that the interval for those was different. Fifty-five physicians (95%) answered that there was no difference in the surveillance time interval for patients according to location (colon vs. rectum); and three (5%) said that there was a difference.
Sixty-four physicians (80%) answered that there was no difference in the follow-up interval between surgery and surveillance of patients with stage II or III CRC.
Thirty-two (49%) and 31 physicians (48%) monitored patients with stage I every 3 and 6 months using outpatient visits and laboratory examinations—including tumor markers, CBC, and blood chemistries—for the first year, respectively.
After 2 years, most physicians monitored patients every 6 months or 1 year. By 3 years, over half of physicians monitored them every 1 year. Of physicians monitoring patients in stage II/III, 45 (65%) and 22 (32%) of the physicians did so every 3 and 6 months for the first year. During the third year, 48 (69%) of physicians monitored patients every 6 months; and 12 (17%) monitored them every year. From 3 years on, most physicians monitored patients every 6 months. For stage IV, 69 of physicians (96%) monitored patients every 3 months for the first year. During the third year, 33 (46%) and 36 (50%) physicians monitored patients every 3 and 6 months. After the third year, about third-quarter of physicians monitored patients every 6 months (Table 2).
Forty-two physicians (66%) monitored patients with stage I CRC every 6 months, and 12 (19%) monitored them every 1 year for the first year. After 3 years, all physicians answered that they monitored patients every 6 months or 1 year. For stage II/III patients, 42 physicians monitored them every 6 months for the first 3 years. From the fourth years on, over half of physicians monitored patients every year. For stage IV patients, 51 (70%) and 21 (29%) physicians monitored patients every 3 and 6 months, respectively, for the first year. From the third years on, most physicians monitored patients every 6 months (Table 3).
Twenty-eight physicians (54%) monitored patients with stage I CRC every 6 months. From 3 years on, all physicians monitored them either every 6 months or 1 year. For stage II/III, 31 (52%) and 18 (30%) physicians monitored patients every 6 months or every year during the first year, respectively. By 3 years, most physicians monitored patients either every 6 months or every year. For stage IV patients, 35 physicians (53%) monitored patients every 3 months, and 22 (33%) monitored them every 6 months, during the first year. From 2 years on, proportionally most of the physicians monitored patients every 6 months (Table 4).
Thirty-six physicians (57%) answered that they monitored patients with stage I; 36 (54%) monitored patients with stage II/III; and 37 (53%) monitored patients with stage IV by colonoscopy in the first year after surgery (Fig. 2).
By PET/CT and a bone scan, 54 physicians (92%) answered that they did not monitor patients with stage I CRC. Fifty-four (88%) and 56 (86%) physicians answered that there was no need to monitor patients with stage II by PET/CT and bone scan, respectively. Fifty-three (84%) and 56 (82%) of physicians answered that there was no need to monitor patients with stage IV using PET/CT and bone scan, respectively.
Intensive postoperative surveillance could improve overall survival and increase the early detection of asymptomatic recurrences. Then, it would make curative surgery attempts for patients with recurrence [6]. The guidelines issued by most expert groups—including the European Society for Medical Oncology (ESMO) [7,8], the American Society of Clinical Oncology (ASCO) [9], and the National Comprehensive Cancer Network (NCCN) [10,11]—recommend intensive postoperative surveillance of patients with stage III CRC and of those at high risk for stage II CRC.
A history, physical examination, and carcinoembryonic antigen (CEA) test should be performed every 3 to 6 months for 2 years and then every 6 months for 5 years for those with a need for the most intensive form of postoperative surveillance. Contrast-enhanced CT scans of the chest and abdominopelvic region are recommended for every 6 to 12 months for up to 5 years [8-10]. Some evaluations are performed more frequently based on the NCCN guidelines during surveillance of patients with stage IV CRC and no evidence of disease after curative intent surgery. Stage IV patients should undergo chest and abdominopelvic CT scans every 3 to 6 months in the first 2 years after adjuvant treatment and then every 6 to 12 months thereafter for up to 5 years [10]. Chest and abdominopelvic CT scans are also performed more frequently in patients with stages I to IV rectal cancer [11]. In addition, a proctoscopy is recommended for every 3 to 6 months for the first 2 years and then every 6 months for 5 years thereafter in patients with for rectal cancer treated only by transanal excision. This survey showed the Korean postoperative strategy in patients with CRC after curative surgery. About 90% of physicians preferred monitoring patients with stage II every 3 to 6 months for the first 2 years and then with laboratory tests every 6 months or 1 year. More than 20% of the physicians preferred monitoring patients more frequently by abdominopelvic CT for the first 2 years; and a similar result was observed for chest CT. This frequent image monitoring is more obvious during the surveillance of patients with stage IV CRC.
Routine CEA monitoring and CT scans are not recommended beyond 5 years, regardless of stage. The ASCO/Cancer Care Ontario and ESMO guidelines recommend abdominal and chest CT scans for 3 years [8,9]; NCCN recommends these every 5 years. Korean physicians tend to perform scans for more than 5 years; as about 23% of them considered scans for stage II, and about 45% conserved them for patients with stage IV. Studies of the relationship between tumor location and recurrence pattern have found that cancers of the left colon and rectum are likely to progress more slowly and that recurrence occurs late more frequently in those tumors [5,12]. Actually, among locations for CRC, rectal cancer showed the highest incidence of 45.5%, followed by distal colon cancer at 27.2% and proximal colon cancer at 20.9% in Korea [13]. This late recurrence may affect the duration of postoperative surveillance. In addition, a comparative study of recurrence patterns within and beyond 5 years after surgery showed that more tumors expanded growth with well-differentiated histology but no lymph node metastasis beyond 5 years after surgery in patients with lower preoperative serum CEA levels [14]. The result that patients with these relatively favorable factors need longer surveillance beyond 5 years might impact physician’s preference regarding surveillance duration. Lastly, patients who had metachronous oligometastasis on the liver or lung would improve survival through metastatectomy, which could also be the reason of a longer duration of postoperative surveillance. However, although a late recurrence is detected early, the possible harm from radiation exposure due to repeated CT scans, psychological stress from surveillance visits and scans, and the stress of false-positive results should be considered.
Another goal of posttreatment CRC surveillance is to identify new metachronous neoplasms. Although use of posttreatment surveillance colonoscopy does not improve survival through early detection of recurrence of the original CRC, it has a benefit for identifying and removing metachronous polyps. Data show that patients who have undergone curative resection for CRC have an increased risk of developing a second cancer, particularly in the first 2 years after resection of the initial primary cancer, with an incidence rate of 0.7% over this interval [15]. Colonoscopy is mainly recommended 1 year after resection. Repeat colonoscopy is typically recommended at 3 years and then every 5 years. Although many physicians in our survey used surveillance colonoscopy during the first year, the proportions of colonoscopies performed during years 2, 3, and 5 years were similar, regardless of tumor stage.
Some limitations of our study should be mentioned. The sample size was small and may have limited the statistical power. Our estimates of practice patterns were based entirely on self-report. Most cancer specialists in Korea are in academic practices and this was reflected in our sample, but follow-up may differ in general practice. Nevertheless, our study is the first to assess real world Korean physicians’ policies for postoperative CRC surveillance. Our results will help to explore and establish Korean physicians’ policies for postoperative CRC surveillance.
In summary, Korean physicians mostly followed up CRC intensively with laboratory exams and CT scans; although a small proportion preferred monitoring patients by CT more frequently during the first 2 years. The physicians tended to prefer monitoring these patients for more than 5 years, particularly those with advanced-stage disease. Many physicians used surveillance colonoscopy during the first year, but the proportions of colonoscopies performed during year 2, 3, and 5 were similar, regardless of tumor stage.
1. Postoperative surveillance of colorectal cancer (CRC) could improve survival through the early detection of recurrences and curative surgery.
2. Korean physicians tended to prefer monitoring advanced stage CRC patients for more than 5 years.
3. The physicians mostly followed up CRC using intensive postoperative surveillance.
Table 1.
Physicians’ surveillance roles
| Total | Surgeon | Medical oncologist | Gastroenterologist | Radiation oncologist | Othera | |
|---|---|---|---|---|---|---|
| Stage I | 70 (100) | 65 (93) | 4 (6) | 19 (27) | 0 | 1 (1) |
| Stage II | 71 (100) | 64 (90) | 16 (23) | 12 (17) | 1 (1) | 1 (1) |
| Stage III | 72 (100) | 57 (79) | 34 (47) | 10 (14) | 5 (7) | 1 (1) |
| Stage IV | 72 (100) | 39 (54) | 50 (69) | 12 (17) | 3 (4) | 1 (1) |
Table 2.
Time interval of postoperative laboratory surveillance, according to stage
Table 3.
Time interval of postoperative abdominopelvic computed tomography surveillance, according to stage
Table 4.
Time interval of postoperative chest computed tomography surveillance, according to stage
REFERENCES
1. Jung KW, Won YJ, Kong HJ, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2012. Cancer Res Treat 2015;47:127–141.




2. Primrose JN, Perera R, Gray A, et al. Effect of 3 to 5 years of scheduled CEA and CT follow-up to detect recurrence of colorectal cancer: the FACS randomized clinical trial. JAMA 2014;311:263–270.


3. Rodriguez-Moranta F, Salo J, Arcusa A, et al. Postoperative surveillance in patients with colorectal cancer who have undergone curative resection: a prospective, multicenter, randomized, controlled trial. J Clin Oncol 2006;24:386–393.


4. Sargent D, Sobrero A, Grothey A, et al. Evidence for cure by adjuvant therapy in colon cancer: observations based on individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol 2009;27:872–877.



5. Cho YB, Chun HK, Yun HR, Lee WS, Yun SH, Lee WY. Clinical and pathologic evaluation of patients with recurrence of colorectal cancer five or more years after curative resection. Dis Colon Rectum 2007;50:1204–1210.


6. Pita-Fernandez S, Alhayek-Ai M, Gonzalez-Martin C, Lopez-Calvino B, Seoane-Pillado T, Pertega-Diaz S. Intensive follow-up strategies improve outcomes in nonmetastatic colorectal cancer patients after curative surgery: a systematic review and meta-analysis. Ann Oncol 2015;26:644–656.



7. Glimelius B, Tiret E, Cervantes A, Arnold D, ESMO Guidelines Working Group. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24 Suppl 6:vi81–vi88.


8. Labianca R, Nordlinger B, Beretta GD, et al. Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24 Suppl 6:vi64–vi72.



9. Meyerhardt JA, Mangu PB, Flynn PJ, et al. Follow-up care, surveillance protocol, and secondary prevention measures for survivors of colorectal cancer: American Society of Clinical Oncology clinical practice guideline endorsement. J Clin Oncol 2013;31:4465–4470.


10. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in oncology: colon cancer. v2. 2016 [Internet] Fort Washington (PA): National Comprehensive Cancer Network, c2017 [cited 2017 Aug 18]. Available from: https://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
11. National Comprehensive Cancer Network. CCN Clinical Practice Guidelines in oncology: rectal cancer. v1. 2016 [Internet] Fort Washington (PA): National Comprehensive Cancer Network, c2017 [cited 2017 Aug 18]. Available from: https://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
12. Eisenberg B, Decosse JJ, Harford F, Michalek J. Carcinoma of the colon and rectum: the natural history reviewed in 1704 patients. Cancer 1982;49:1131–1134.


13. Shin A, Kim KZ, Jung KW, et al. Increasing trend of colorectal cancer incidence in Korea, 1999-2009. Cancer Res Treat 2012;44:219–226.







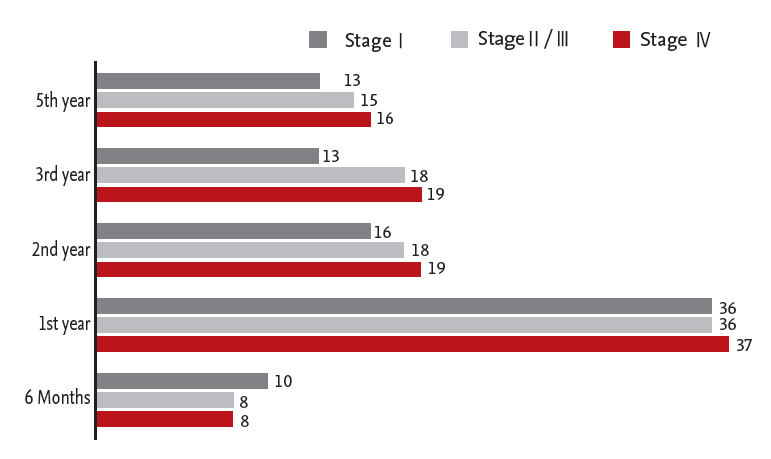
 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



