 |
 |
| Korean J Intern Med > Volume 35(4); 2020 > Article |
|
Abstract
Background/Aims
Methods
Results
Conclusions
Acknowledgments
Supplementary Materials
Supplementary Table 1.
Supplementary Table 2.
Supplementary Table 3.
Supplementary Table 4.
Figure 1.
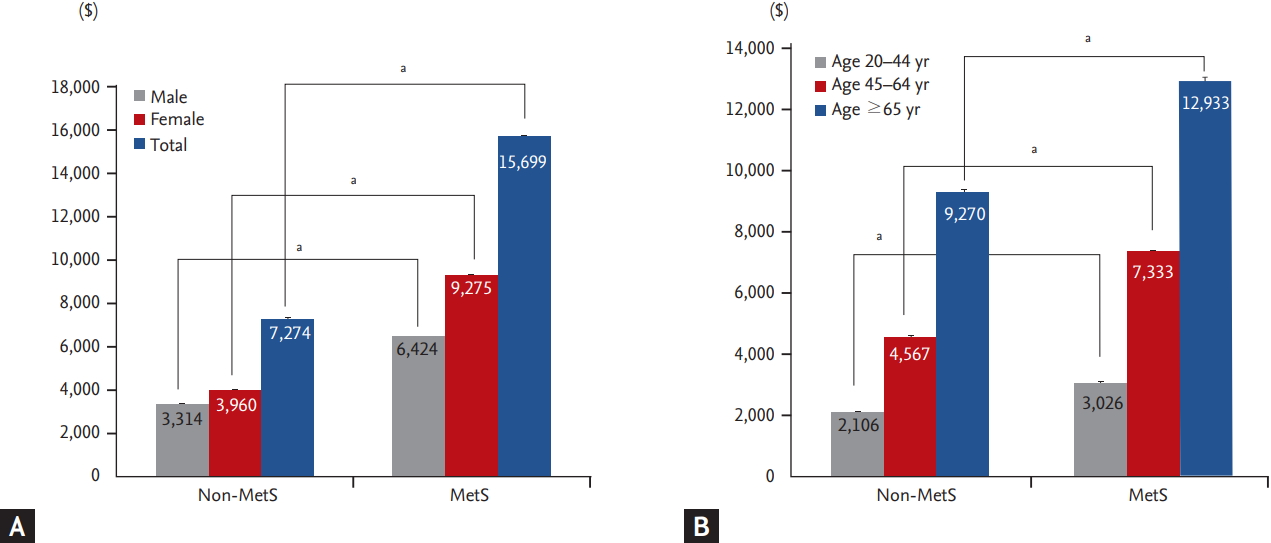
Figure 2.
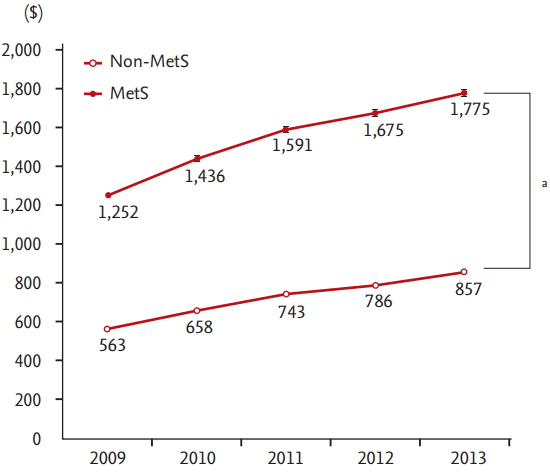
Figure 3.
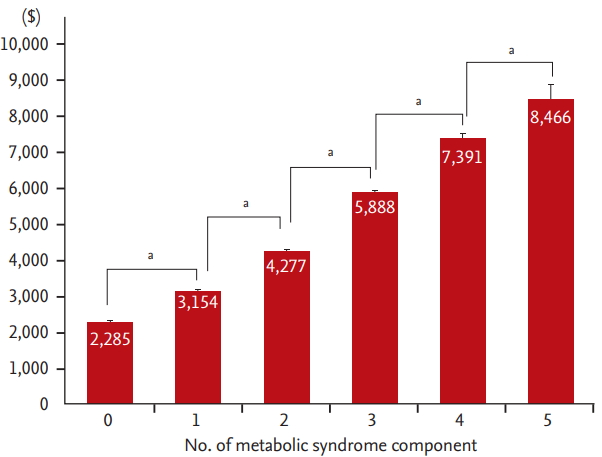
Table 1.
Values are presented as number (%) or geometric mean (95% confidence interval). Percentages may not sum to 100% because of missing data. Abdominal obesity, waist circumference ≥ 90 cm in men and ≥ 85 cm in women; hypertriglyceridemia, serum triglyceride ≥ 150 mg/dL; low HDL-C level, HDL-C < 40 mg/dL in men and < 50 mg/dL in women; high blood pressure, blood pressure ≥ 130/85 mmHg or current use of antihypertensive medication; hyperglycemia, serum glucose ≥ 100 mg/dL or current use of medication for diabetes.
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBG, fasting blood glucose; TC, total cholesterol; TG, triglyceride; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; Cr, creatinine; AST, aspartate transaminase; ALT, alanine aminotransferase; γGT, gamma-glutamyl transpeptidase; NCEP-ATP, National Cholesterol Education Program-Adult Treatment Panel.
Table 2.
| Variable |
Simple regression model |
Multiple regression models I through VI |
Multiple regression model VII |
Multiple regression model VIII |
||||
|---|---|---|---|---|---|---|---|---|
| Parameter estimation, USD | p value | Parameter estimation, USD | p value | Parameter estimation, USD | p value | Parameter estimation, USD | p value | |
| Metabolic syndrome | 4,123 | < 0.001 | 2,401 | < 0.001 | 850 | < 0.001 | 561 | < 0.001 |
| Abdominal obesity | 2,818 | < 0.001 | 1,335 | < 0.001 | 422 | < 0.001 | 487 | < 0.001 |
| Hypertriglyceridemia | 2,401 | < 0.001 | 1,529 | < 0.001 | 701 | < 0.001 | 557 | < 0.001 |
| Low HDL-C level | 1,588 | < 0.001 | 581 | 0.601 | 33 | 0.485 | 29 | 0.549 |
| High blood pressure | 3,397 | < 0.001 | 1,833 | < 0.001 | 1,182 | < 0.001 | 1,033 | < 0.001 |
| Hyperglycemia | 2,736 | < 0.001 | 1,512 | < 0.001 | 803 | < 0.001 | 750 | < 0.001 |
| Age | 4,147 | < 0.001 | a | < 0.001 | 3,200 | < 0.001 | 2,966 | < 0.001 |
| Female gender | 1,267 | < 0.001 | b | < 0.001 | 706 | < 0.001 | 667 | < 0.001 |
| Intercept | - | - | c | < 0.001 | –2,425 | < 0.001 | –2,031 | < 0.001 |
| Cardiac disorders | - | - | - | - | - | - | 3,069 | < 0.001 |
| Stroke | - | - | - | - | - | - | 4,145 | < 0.001 |
Multiple regressions models I through VI include age, sex, household income level, smoking status, drinking level, exercise status, and each individual metabolic syndrome component. Multiple regression model VII includes all components of metabolic syndrome and controls for age, sex, household income level, smoking status, drinking level, and exercise status. Multiple regression model VIII adds controls for cardiac disorders and stroke to model VII. Abdominal obesity, waist circumference ≥ 90 cm in men and ≥ 85 cm in women; hypertriglyceridemia, serum triglyceride ≥ 150 mg/dL; low HDL-C level, HDL-C < 40 mg/dL in men and < 50 mg/dL in women; high blood pressure, blood pressure ≥ 130/85 mmHg or current use of antihypertensive medication; hyperglycemia, serum glucose ≥ 100 mg/dL or current use of medication for diabetes.
USD, US dollar; HDL-C, high density lipoprotein cholesterol.
a Individual parameter estimates for age were as follows: model I (metabolic syndrome [MetS]), 3,493 USD; model II (abdominal obesity), 3,814 USD; model III (hypertriglyceridemia), 3,749 USD; model IV (low HDL-C), 3,951 USD; model V (high blood pressure), 1,833 USD; and model VI (hyperglycemia), 3,711 USD.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement 1
Supplement 1 Print
Print



